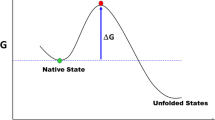
Abstract
Using thermal adaptation of enzymes as an example, we have proposed a molecular and thermodynamic model for protein adaptation. Key concepts: (1) The working mechanism of enzymatic reactions is not altered in protein adaptation, but the activity of the adapted enzyme is expressed under altered conditions. (2) The alteration of protein conformational stability induced by gene mutation is the fundamental cause of protein adaptation. (3) The population change in active conformations of enzymes induced by protein conformational stability in different temperature ranges is the major cause of protein adaptation. (4) The features of enzyme adaptation must be analyzed or judged by two different aspects: local population change in active conformations near a critical level of an environmental factor; and the position of the whole active conformational curve in the gradient of an environmental factor. (5) Protein adaptation represents a specific mechanism for protein regulation. Several other aspects of protein adaptation are also discussed and reviewed, and specific examples are given of enzymes showing particular types of adaptation.








Similar content being viewed by others
References
Åqvist J, Sočan J, Purg M (2020) Hidden conformational states and strange temperature optima in enzyme catalysis. Biochemistry 59(40):3844–3855
Arcus VL, Prentice EJ, Hobbs JK, Mulholland AJ, Van der Kamp MW, Pudney CR, Parker EJ, Schipper LA (2016) On the temperature dependence of enzyme-catalyzed rates. Biochemistry 55(12):1681–1688
Arnold FH, Wintrode PL, Miyazaki K, Gershenson A (2001) How enzymes adapt: lessons from directed evolution. Trends Biochem Sci 26:100–106
Becktel WJ, Schellman JA (1987) Protein stability curves. Biopolymers 26:1859–1877
Beers JM, Jayasundara N (2015) Antarctic notothenioid fish: what are the future consequences of “losses” and “gains” acquired during long-term evolution at cold and stable temperatures? J Exp Biol 218:1834–1845
Beney L, Gervais P (2001) Influence of the fluidity of the membrane on the response of microorganisms to environmental stresses. Appl Microbiol Biotechnol 57(1):34–42
Berger VJ, Kharazova AD (1997) Mechanisms of salinity adaptations in marine molluscs. Interactions and adaptation strategies of marine organisms. Springer, Dordrecht, pp 115–126
Bismuto E, Nucci R, Febbraio F, Tanfani F, Gentile F, Briante R, Scire A, Bertoli E, Amodeo P (2004) Effects induced by mono-and divalent cations on protein regions responsible for thermal adaptation in β-glycosidase from Sulfolobus solfataricus. Eur Biophys J 33(1):38–49
Bloom JD, Meyer MM, Meinhold P, Otey CR, MacMillan D, Arnold FH (2005) Evolving strategies for enzyme engineering. Curr Opin Struc Biol 15:447–452
Boehr DD, Nussinov R, Wright PE (2009) The role of dynamic conformational ensembles in biomolecular recognition. Nat Chem Biol 5:789–796
Cedervall P, Aulabaugh A, Geoghegan KF, McLellan TJ, Pandit J (2015) Engineered stabilization and structural analysis of the autoinhibited conformation of PDE4. Proc Natl Acad Sci USA 112(12):E1414–E1422
Collins T, Roulling F, Piette F, Marx JC, Feller G, Gerday C, D’Amico S (2008) Fundamentals of cold-adapted enzymes. Psychrophiles: from biodiversity to biotechnology. Springer, Berlin, pp 211–227
Daniel RM, Peterson ME, Danson MJ, Price NC, Kelly SM, Monk CR, Weinberg CS, Oudshoorn ML, Lee CK (2010) The molecular basis of the effect of temperature on enzyme activity. Biochem J 425:353–360
De Simone A, Aprile FA, Dhulesia A, Dobson CM, Vendruscolo M (2015) Structure of a low-population intermediate state in the release of an enzyme product. Elife 4:e02777
Elias M, Wieczorek G, Rosenne S, Tawfik DS (2014) The universality of enzymatic rate-temperature dependency. Trends Biochem Sci 39:1–7
Etchebest C, Benros C, Bornot A, Camproux AC, De Brevern AG (2007) A reduced amino acid alphabet for understanding and designing protein adaptation to mutation. Eur Biophys J 36(8):1059–1069
Falsone SF, Leptihn S, Osterauer A, Haslbeck M, Buchner J (2004) Oncogenic mutations reduce the stability of SRC kinase. J Mol Biol 344:281–291
Fariselli P, Olmea O, Valencia A, Casadio R (2001) Prediction of contact maps with neural networks and correlated mutations. Protein Eng 14:835–843
Fersht AR (1977) Enzyme structure and mechanism. WH Freeman, New York
Fersht AR, Serrano L (1993) Principles of protein stability derived from protein engineering experiments. Curr Opin Struct Biol 3(1):75–83
Fields PA, Somero GN (1998) Hot spots in cold adaptation: localized increases in conformational flexibility in lactate dehydrogenase A4 orthologs of Antarctic notothenioid fishes. Proc Natl Acad Sci USA 95:11476–11481
Fields PA, Dong Y, Meng X, Somero GN (2015) Adaptations of protein structure and function to temperature: there is more than one way to “skin a cat.” J Exp Biol 218:1801–1811
Gerday C, Aittaleb M, Arpigny JL, Baise E, Chessa JP, Garsoux G, Petrescu I, Feller G (1997) Psychrophilic enzymes: a thermodynamic challenge. Biochim Biophys Acta (BBA) Protein Struct Mol Enzymol 1342(2):119–131
Gether U, Seifert R, Ballesteros JA, Sanders-Bush E, Weinstein H, Kobilka BK (1997) structural instability of a constitutively active G protein-coupled receptor agonist-independent activation due to conformational flexibility. J Biol Chem 272:2587–2590
Ghadessy FJ, Ramsay N, Boudsocq F, Loakes D, Brown A, Iwai S, Vaisman A, Woodgate R, Holliger P (2004) Generic expansion of the substrate spectrum of a DNA polymerase by directed evolution. Nat Biotechnol 22:755–759
Guerois R, Nielsen JE, Serrano L (2002) Predicting changes in the stability of proteins and protein complexes: a study of more than 1000 mutations. J Mol Biol 320:369–387
Hajdú I, Bőthe C, Szilágyi A, Kardos J, Gál P, Závodszky P (2008) Adjustment of conformational flexibility of glyceraldehyde-3-phosphate dehydrogenase as a means of thermal adaptation and allosteric regulation. Eur Biophys J 37(7):1139–1144
Hammes GG (2002) Multiple conformational changes in enzyme catalysis. Biochemistry 41:8221–8228
Hardman MJ (1981) The rate-determining step in the liver alcohol dehydrogenase-catalysed reduction of acetaldehyde is an isomerization of the enzyme. Biochem J 195:773–774
Harris H (1971) Polymorphism and protein evolution. The neutral mutation-random drift hypothesis. J Med Genet 8:444–452
Hernandez G, Jenney FE, Adams MWW, LeMaster DM (2000) Millisecond time scale conformational flexibility in a hyperthermophile protein at ambient temperature. Proc Natl Acad Sci USA 97:3166–3170
Hiller R, Zhou ZH, Adams MW, Englander SW (1997) Stability and dynamics in a hyperthermophilic protein with melting temperature close to 200 C. Proc Natl Acad Sci USA 94(21):11329–11332
Honaker MT, Acchione M, Sumida JP, Atkins WM (2011) Ensemble perspective for catalytic promiscuity calorimetric analysis of the active site conformational landscape of a detoxification enzyme. J Biol Chem 286:42770–42821
Isaksen GV, Åqvist J, Brandsdal BO (2014) Protein surface softness is the origin of enzyme cold-adaptation of trypsin. PLoS Comput Biol 10:e1003813
Jaenicke R (1991) Protein stability and molecular adaptation to extreme conditions. EJB reviews. Springer, Berlin, pp 291–304
Jencks WP (1975) Binding energy, specificity, and enzymic catalysis: the circe effect. Adv Enzymol Relat Areas Mol Biol 43:219–410
Karshikoff A, Nilsson L, Ladenstein R (2015) Rigidity versus flexibility: the dilemma of understanding protein thermal stability. FEBS J 282:3899–3917
Khersonsky O, Roodveldt C, Tawfik DS (2006) Enzyme promiscuity: evolutionary and mechanistic aspects. Curr Opin Chem Biol 10:498–508
Kjelsberg MA, Cotecchia S, Ostrowski J, Caron MG, Lefkowitz RJ (1992) Constitutive activation of the alpha 1B-adrenergic receptor by all amino acid substitutions at a single site. Evidence for a region which constrains receptor activation. J Biol Chem 267:1430–1433
Liang ZX, Tsigos I, Lee T, Bouriotis V, Resing KA, Ahn NG, Klinman JP (2004) Evidence for increased local flexibility in psychrophilic alcohol dehydrogenase relative to its thermophilic homologue. Biochemistry 43:14676–14683
Low PS, Bada JL, Somero GN (1973) Temperature adaptation of enzymes: roles of the free energy, the enthalpy, and the entropy of activation. Proc Natl Acad Sci USA 70(2):430–432
Madern D, Ebel C, Zaccai G (2000) Halophilic adaptation of enzymes. Extremophiles 4(2):91–98
Maisnier-Patin S, Andersson DI (2004) Adaptation to the deleterious effects of antimicrobial drug resistance mutations by compensatory evolution. Res Microbiol 155:360–369
Makhatadze GI, Privalov PL (1995) Energetics of protein structure. Adv Protein Chem 47:307–425
Matthews BW (1993) Structural and genetic analysis of protein stability. Annu Rev Biochem 62:139–160
Nagel ZD, Dong M, Bahnson BJ, Klinman JP (2011) Impaired protein conformational landscapes as revealed in anomalous Arrhenius prefactors. Proc Natl Acad Sci USA 108:10520–10525
Olivares I, Sánchez-Merino V, Martínez MA, Domingo E, López-Galíndez C, Menéndez-Arias L (1999) Second-site reversion of a human immunodeficiency virus type 1 reverse transcriptase mutant that restores enzyme function and replication capacity. J Virol 73(8):6293–6298
Pace CN (1975) The stability of globular proteins. CRC Crit Rev Biochem 3:1–43
Pace CN (1992) Contributions of the hydrophobic effect to globular protein stability. J Mol Biol 226:29–35
Pace CN, Shirley BA, McNutt M, Gajiwala K (1996) Forces contributing to the conformational stability of proteins. FASEB J 10(1):75–83
Page MJ, Cera ED (2006) Role of Na+ and K+ in enzyme function. Physiol Rev 86(4):1049–1092
Pischedda A, Ramasamy KP, Mangiagalli M, Chiappori F, Milanesi L, Miceli C, Pucciarelli S, Lotti M (2018) Antarctic marine ciliates under stress: superoxide dismutases from the psychrophilic Euplotes focardii are cold-active yet heat tolerant enzymes. Sci Rep 8:1–13
Prevost M, Wodak SJ, Tidor B, Karplus M (1991) Contribution of the hydrophobic effect to protein stability: analysis based on simulations of the Ile-96-Ala mutation in barnase. Proc Natl Acad Sci USA 88:10880–10884
Privalov PL (1990) Cold denaturation of protein. Crit Rev Biochem Mol Biol 25:281–306
Rasmussen SG, Jensen AD, Liapakis G, Ghanouni P, Javitch JA, Gether U (1999) Mutation of a highly conserved aspartic acid in the β2 adrenergic receptor: constitutive activation, structural instability, and conformational rearrangement of transmembrane segment 6. Mol Pharm 56:175–184
Saavedra HG, Wrabl JO, Anderson JA, Li J, Hilser VJ (2018) Dynamic allostery can drive cold adaptation in enzymes. Nature 558:324–328
Scheer A, Fanelli F, Costa T, De Benedetti PG, Cotecchia S (1996) Constitutively active mutants of the alpha 1B-adrenergic receptor: role of highly conserved polar amino acids in receptor activation. EMBO J 15:3566–3578
Shukla H, Shukla R, Sonkar A, Pandey T, Tripathi T (2017) Distant Phe345 mutation compromises the stability and activity of Mycobacterium tuberculosis isocitrate lyase by modulating its structural flexibility. Sci Rep 7:1–11
Somero GN (1995) Proteins and temperature. Annu Rev Physiol 57(1):43–68
Stiller JB, Otten R, Häussinger D, Rieder PS, Theobald DL, Kern D (2022) Structure determination of high-energy states in a dynamic protein ensemble. Nature. https://doi.org/10.1038/s41586-022-04468-9
Suzuki T, Yasugi M, Arisaka F, Yamagishi A, Oshima T (2001) Adaptation of a thermophilic enzyme, 3-isopropylmalate dehydrogenase, to low temperatures. Protein Eng 14:85–91
Takagi Y, Taira K (1995) Temperature-dependent change in the rate-determining step in a reaction catalyzed by a hammerhead ribozyme. FEBS Lett 361:273–276
Tattersall GJ, Sinclair BJ, Withers PC, Fields PA, Seebacher F, Cooper CE, Maloney SK (2012) Coping with thermal challenges: physiological adaptations to environmental temperatures. Compr Physiol 2(3):2151–2202
Tokuriki N, Stricher F, Schymkowitz J, Serrano L, Tawfik DS (2007) The stability effects of protein mutations appear to be universally distributed. J Mol Biol 369:1318–1332
Traut TW (1994) Dissociation of enzyme oligomers: a mechanism for allosteric regulation. Crit Rev Biochem Mol Biol 29:125–163
Truhlar DG, Kohen A (2001) Convex Arrhenius plots and their interpretation. Proc Natl Acad Sci USA 98(3):848–851
Vieille C, Zeikus GJ (2001) Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev 65:1–43
Vogt G, Woell S, Argos P (1997) Protein thermal stability, hydrogen bonds, and ion pairs. J Mol Biol 269:631–643
Vyazovkin S (2000) Kinetic concepts of thermally stimulated reactions in solids: a view from a historical perspective. Int Rev Phys Chem 19(1):45–60
Warshel A, Sharma PK, Kato M, Parson WW (2006) Modeling electrostatic effects in proteins. Biochim Biophys Acta -Proteins and Proteomics 1764:1647–1676
Worth CL, Gong S, Blundell TL (2009) Structural and functional constraints in the evolution of protein families. Nat Rev Mol Cell Biol 10:709–720
Xie Y, An J, Yang G, Wu G, Zhang Y, Cui L, Feng Y (2014) Enhanced enzyme kinetic stability by increasing rigidity within the active site. J Biol Chem 289:7994–8006
Zavodszky P, Kardos J, Svingor PGA (1998) Adjustment of conformational flexibility is a key event in the thermal adaptation of proteins. Proc Natl Acad Sci USA 95:7406–7411
Zhao Q (2009) Protein thermodynamic structure. IUBMB Life 61:600–606
Zhao Q (2011) Dynamic model of enzymes action. Protein Pept Lett 18:92–99
Zhao Q (2012) Partition function of the protein conformational state. J Comput Theor Nanos 9:745–751
Zhao Q (2015) A thermodynamic and theoretical view for enzyme regulation. Biochem Mosc 80:1–7
Zhao Q (2016) Classification of enzyme regulators within thermodynamic model of enzyme regulation. Mol Enz Drug Tar 2:14
Zhao Q (2017a) The origin of natural order: an axiomatic theory of biology. World Scientific Press, Singapore
Zhao Q (2017b) On the indirect relationship between protein dynamics and enzyme activity. Prog Biophys Mol Biol 125:52–60
Zhao Q (2018) Physical characteristics of complex thermal system and working mechanism of enzymes. Sci Sin Vitae 48:650–661
Zhao Q (2022) Revised equation of enzymatic kinetics and thermodynamic mechanisms for directed evolution of enzymes. Int J Chem Kinet. https://doi.org/10.1002/kin.21558
Zhao L, Vogt PK (2008) Helical domain and kinase domain mutations in p110alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc Natl Acad Sci USA 105:2652–2657
Zhou H, Zhou Y (2002) Distance-scaled, finite ideal-gas reference state improves structure-derived potentials of mean force for structure selection and stability prediction. Protein Sci 11:2714–2726
Acknowledgements
I would like to thank Professor Robert Gilbert (Wellcome Trust Centre for Human Genetics, University of Oxford, UK) for his help in the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Q. Molecular and thermodynamic mechanisms for protein adaptation. Eur Biophys J 51, 519–534 (2022). https://doi.org/10.1007/s00249-022-01618-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-022-01618-9