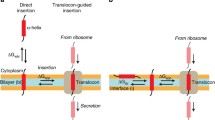
Abstract
Hydrophobic amino acids are abundant in transmembrane (TM) helices of membrane proteins. Charged residues are sparse, apparently due to the unfavorable energetic cost of partitioning charges into nonpolar phases. Nevertheless, conserved arginine residues within TM helices regulate vital functions, such as ion channel voltage gating and integrin receptor inactivation. The energetic cost of arginine in various positions along hydrophobic helices has been controversial. Potential of mean force (PMF) calculations from atomistic molecular dynamics simulations predict very large energetic penalties, while in vitro experiments with Sec61 translocons indicate much smaller penalties, even for arginine in the center of hydrophobic TM helices. Resolution of this conflict has proved difficult, because the in vitro assay utilizes the complex Sec61 translocon, while the PMF calculations rely on the choice of simulation system and reaction coordinate. Here we present the results of computational and experimental studies that permit direct comparison with the Sec61 translocon results. We find that the Sec61 translocon mediates less efficient membrane insertion of Arg-containing TM helices compared with our computational and experimental bilayer-insertion results. In the simulations, a combination of arginine snorkeling, bilayer deformation, and peptide tilting is sufficient to lower the penalty of Arg insertion to an extent such that a hydrophobic TM helix with a central Arg residue readily inserts into a model membrane. Less favorable insertion by the translocon may be due to the decreased fluidity of the endoplasmic reticulum (ER) membrane compared with pure palmitoyloleoyl-phosphocholine (POPC). Nevertheless, our results provide an explanation for the differences between PMF- and experiment-based penalties for Arg burial.







Similar content being viewed by others
References
Berendsen HJC, van der Spoel D, van Drunen R (1995) GROMACS: a new message-passing parallel molecular dynamics implementation. Comput Phys Commun 91:43–56
Capponi S, Heyden M, Bondar AN, Tobias DJ, White SH (2015) Anomalous behavior of water inside the SecY translocon. Proc Natl Acad Sci USA 112(29):9016–9021
Cymer F, von Heijne G, White SH (2015) Mechanisms of integral membrane protein insertion and folding. J Mol Biol 427(5):999–1022
de Planque MRR et al (1999) Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane α-helical peptides. Biochemistry 274(30):20839–20846
Doherty T, Su Y, Hong M (2010) High-resolution orientation and depth of insertion of the voltage-sensing S4 helix of a potassium channel in lipid bilayers. J Mol Biol 401:642–652
Dorairaj S, Allen TW (2007) On the thermodynamic stability of a charged arginine side chain in a transmembrane helix. Proc Natl Acad Sci USA 104(12):4943–4948
Fleming PJ, Freites JA, Moon CP, Tobias DJ, Fleming KG (2011) Outer membrane phospholipase A in phospholipid bilayers: a model system for concerted computational and experimental investigations of amino acid side chain partitioning into lipid bilayers. Biochim Biophys Acta 1818:126–134
Freites JA, Tobias DJ, von Heijne G, White SH (2005) Interface connections of a transmembrane voltage sensor. Proc Natl Acad Sci USA 102(42):15059–15064
Greenfield NJ (2007) Using circular dichroism collected as a function of temperature to determine the thermodynamics of protein unfolding and binding interactions. Nat Protoc 1:2527–2535
Gumbart J, Roux B (2012) Determination of membrane-insertion free energies by molecular dynamics simulations. Biophys J 102:795–801
Gumbart J, Chipot C, Schulten K (2011) Free-energy cost for translocon-assisted insertion of membrane proteins. Proc Natl Acad Sci USA 108:3596–3601
Hessa T et al (2005a) Recognition of transmembrane helices by the endoplasmic reticulum translocon. Nature 433:377–381
Hessa T, White SH, von Heijne G (2005b) Membrane insertion of a potassium channel voltage sensor. Science 307:1427
Hessa T et al (2007) The molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature 450:1026–1030
Hristova K et al (1999) An amphipathic α-helix at a membrane interface: a structural study using a novel x-ray diffraction method. J Mol Biol 290:99–117
Hristova K, Dempsey CE, White SH (2001) Structure, location, and lipid perturbations of melittin at the membrane interface. Biophys J 80:801–811
Jayasinghe S, Hristova K, White SH (2001) Energetics, stability, and prediction of transmembrane helices. J Mol Biol 312(5):927–934
Jiang YX et al (2003) X-ray structure of a voltage-dependent K+ channel. Nature 423:33–41
Johansson ACV, Lindahl E (2008) Position-resolved free energy of solvation for amino acids in lipid membranes from molecular dynamics simulations. Proteins 70(1332–1344):15
Johansson AC, Lindahl E (2009a) The role of lipid composition for insertion and stabilization of amino acids in membranes. J Chem Phys 130:185101
Johansson ACV, Lindahl E (2009b) Titratable amino acid solvation in lipid membranes as a function of protonation state. J Phys Chem 113:245–253
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79(2):926–935
Jorgensen WL, Maxwell DS, Tirado-Rives J (1996) Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc 118:11225–11236
Kim C et al (2012) Basic amino-acid side chains regulate transmembrane integrin signalling. Nature 481:209–213
Krepkiy D et al (2009) Structure and hydration of membranes embedded with voltage-sensing domains. Nature 462:473–479
Ladokhin AS, White SH (1999) Folding of amphipathic alpha-helices on membranes: energetics of helix formation by melittin. J Mol Biol 285(4):1363–1369
MacCallum JL, Tieleman DP (2011) Hydrophobicity scales: a thermodynamic looking glass into lipid-protein interactions. Trends Biochem Sci 36:653–662
MacCallum JL, Bennett WFD, Tieleman DP (2007) Partitioning of amino acid side chains into lipid bilayers: Results from computer simulations and comparison to experiment. J Gen Physiol 129(5):371–377
MacCallum JL, Bennett WFD, Tieleman DP (2008) Distribution of amino acids in a lipid bilayer from computer simulations. Biophys J 94:3393–3404
MacCallum JL, Bennett WFD, Tieleman DP (2011) Transfer of arginine into lipid bilayers is nonadditive. Biophys J 101:110–117
Moon CP, Fleming KG (2011) Side-chain hydrophobicity scale derived from transmembrane protein folding into lipid bilayers. Proc Natl Acad Sci USA 108:10174–10177
Pace CN, Vajdos F, Fee L, Grimsley G, Gray T (1995) How to measure and predict the molar absorption coefficient of a protein. Protein Sci 4(11):2411–2423
Radzicka A, Wolfenden R (1988) Comparing the polarities of the amino acids: side-chain distribution coefficients between the vapor phase, cyclohexane, 1-octanol, and neutral aqueous solution. Biochemistry 27(5):1664–1670
Rychkova A, Vicatos S, Warshel A (2010) On the energetics of translocon-assisted insertion of charged transmembrane helices into membranes. Proc Natl Acad Sci USA 107:17598–17603
Schow EV et al (2011) Arginine in membranes: the connection between molecular dynamics simulations and translocon-mediated insertion experiments. J Membr Biol 239:35–48
Segrest JP, Deloof H, Dohlman JG, Brouillette CG, Anantharamaiah GM (1990) Amphipathic helix motif—classes and properties. Proteins 8:103–117
Ulmschneider JP, Ulmschneider MB (2009) United atom lipid parameters for combination with the optimized potentials for liquid simulations all-atom force field. J Chem Theory Comput 5:1803–1813
Ulmschneider JP, Doux JP, Killian JA, Smith JC, Ulmschneider MB (2009) Peptide partitioning and folding into lipid bilayers. J Chem Theory Comput 5(9):2202–2205
Ulmschneider MB, Smith JC, Ulmschneider JP (2010a) Peptide partitioning properties from direct insertion studies. Biophys J 98:L60–L62
Ulmschneider MB, Doux JP, Killian JA, Smith JC, Ulmschneider JP (2010b) Mechanism and kinetics of peptide partitioning into membranes from all-atom simulations of thermostable peptides. J Am Chem Soc 132(10):3452–3460
Ulmschneider JP, Smith JC, White SH, Ulmschneider MB (2011) In silico partitioning and transmembrane insertion of hydrophobic peptides under equilibrium conditions. J Am Chem Soc 133:15487–15495
Ulmschneider MB et al (2014) Spontaneous transmembrane helix insertion thermodynamically mimics translocon-guided insertion. Nat Commun 5:4863
Vorobyov I, Li L, Allen TW (2008) Assessing atomistic and coarse-grained force fields for protein-lipid interactions: the formidable challenge of an ionizable side chain in a membrane. J Phys Chem 112:9588–9602
Vostrikov VV, Hall BA, Greathouse DV, Koeppe RE II, Sansom MSP (2010) Changes in transmembrane helix alignment by arginine residues revealed by solid-state NMR experiments and coarse-grained MD simulations. J Am Chem Soc 132:5803–5811
Wee CL, Chetwynd A, Sansom MSP (2011) Membrane insertion of a voltage sensor helix. Biophys J 100:410–419
White SH, Wimley WC (1999) Membrane protein folding and stability: physical principles. Annu Rev Biophys Biomol Struct 28:319–365
Wimley WC, White SH (1996) Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat Struct Biol 3(10):842–848
Wimley WC, White SH (2000) Designing transmembrane α-helices that insert spontaneously. Biochemistry 39(15):4432–4442
Wimley WC, Creamer TP, White SH (1996) Solvation energies of amino acid sidechains and backbone in a family of host-guest pentapeptides. Biochemistry 35:5109–5124
Wu Y, Huang HW, Olah GA (1990) Method of oriented circular dichroism. Biophys J 57(4):797–806
Yoo J, Cui Q (2008) Does arginine remain protonated in the lipid membrane? Insights from microscopic pKa calculations. Biophys J Biophys Lett 94:L61–L63
Zhang B, Miller TF III (2010) Hydrophobically stabilized open state for the lateral gate of the Sec translocon. Proc Natl Acad Sci USA 107:5399–5404
Acknowledgements
This research was supported by a Marie Curie International Fellowship to M.B.U., grants from the National Institute of General Medical Science GM74737 (S.H.W.), Program Project GM86685 from NINDS and NIGMS (S.H.W., D.J.T.), NSF grant CHE-0750175 (D.J.T.), and from the European Research Council (ERC-2008-AdG 232648), the Swedish Cancer Foundation, the Swedish Research Council, and the Swedish Foundation for Strategic Research (G.v.H.).
Author information
Authors and Affiliations
Corresponding author
Additional information
“Special Issue: Shining Light on Membrane Proteins”
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ulmschneider, M.B., Ulmschneider, J.P., Freites, J.A. et al. Transmembrane helices containing a charged arginine are thermodynamically stable. Eur Biophys J 46, 627–637 (2017). https://doi.org/10.1007/s00249-017-1206-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-017-1206-x