Abstract
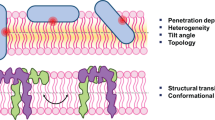
Static and time-resolved fluorescence of tryptophan and ortho-aminobenzoic acid was used to investigate the interaction of the synthetic antimicrobial peptide L1A (IDGLKAIWKKVADLLKNT-NH2) with POPC and POPC:POPG. N-acetylated (Ac-L1A) and N-terminus covalently bonded ortho-aminobenzoic acid (Abz-L1A-W8V) were also used. Static fluorescence and quenching by acrylamide showed that the peptides adsorption to the lipid bilayers was accompanied by spectral blue shift and by a decrease in fluorescence quenching, indicating that the peptides moved to a less polar environment probably buried in the lipidic phase of the vesicles. These results also suggest that the loss of the N-terminus charge allowed deeper fluorophore insertion in the bilayer. Despite the local character of spectroscopic information, conclusions can be drawn about the peptides as a whole. The dynamic behaviors of the peptides are such that the mean intensity lifetimes, the long correlation time and the residual anisotropy at long times increased when the peptides adsorb in lipid vesicles, being larger in anionic vesicles. From the steady-state increase in fluorescence intensity and anisotropy, we observed that the partition coefficient of peptides L1A and its Abz analog in both types of vesicles are higher than the acetylated analog; moreover, the affinity to the anionic vesicle is higher than to the zwitterionic.







Similar content being viewed by others
References
Almeida PF, Pokorny A (2009) Mechanism of antimicrobial, cytolytic and cell-penetrating peptides: from kinetics to thermodynamics. Biochemistry 48:8083–8093
Arbuzova A, Schwarz G (1999) Pore-forming action of mastoparan peptides on liposomes: a quantitative analysis. Biochim Biophys Acta 1420:139–152
Brockman H (1994) Dipole potential of lipid-membranes. Chem Phys Lipids 73:57–79
Castanho MARB, Prieto MJE (1992) Fluorescence study of the macrolide pentaene antibiotic filipin in aqueous solution and in a model system of membranes. Eur J Biochem 207:125–134
Chen Y, Mant CT, Farmer SW, Hancock REW, Vasil ML, Hodges RS (2005) Rational design of alpha-helical antimicrobial peptide with enhanced activity and specificity/therapeutic index. J Biol Chem 280:12316–12329
Clayton AHA, Sawyer WH (1999) Tryptophan rotamer distributions in amphipathic peptides at a lipid surface. Biophys J 76:3235–3242
Coutinho A, Prieto M (1995) Self-association of the polyene antibiotic nistatin in dipalmitoylphosphatidylcholine vesicles: a time-resolved fluorescence study. Biophys J 69:2541–2557
Dathe M, Wieprecht T (1999) Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim Biophys Acta 1462:71–87
Dathe M, Nikolenko H, Meyer J, Beyermann M, Bienert M (2001) Optimization of the antimicrobial activity of magainin peptides by modification of charge. FEBS Lett 501:146–150
dos Santos Cabrera MP, Costa STB, Souza BM, Palma MS, Ruggiero JR, Ruggiero Neto J (2008) Selectivity in the mechanism of action of antimicrobial mastoparan peptide Polybia-MP1. Eur Biophys J 37:879–891
dos Santos Cabrera MP, Alvares DS, Leite NB, Souza BM, Palma MS, Riske KA, Ruggiero Neto J (2011) New insight into the mechanism of action of wasp mastoparan peptides: lytic activity and clustering observed with giant vesicles. Langmuir 27:10805–10813
Engelborghs Y (2001) The analysis of time resolved protein fluorescence in multi-tryptophan proteins. Spectrochim Acta 57:2255–2270
Epand RM, Epand RF (2009) Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim Biophys Acta 1788:289–294
Ferre R, Melo MN, Correia AD, Feliu L, Bardaji E, Planas M, Castanho M (2009) Synergistic effects of the membrane actions of ceropin-melittin antimicrobial hybrid peptide BP100. Biophysical J 96:1815–1827
Gazzara JA, Philips MC, Lund-Katz S, Palgunachari MN, Segrest JP, Anantharamaiah GM, Snow JS (1997) Interaction of class A amphipathic helical peptides with phospholipid unilamellar vesicles. J Lipid Res 38:2134–2146
Goldman C, Pascutti PG, Piquini P, Ito AS (1995) On the contribution of electron transfer reaction to the quenching of tryptophan fluorescence. J Chem Phys 103:10614–10620
Haldar S, Chaudhuri A, Gu H, Koeppe RE, Kombrabail M, Krishnamoorthy G, Chattopadhya A (2012) Membrane Organization and Dynamics of “Inner Pair” and “Outer Pair” Tryptophan Residues in Gramicidin Channels. J Phys Chem B 116:11056–11064
Hancock REW, Sahl H-G (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24:1551–1557
Ito AS, Castrucci AML, Hruby VJ, Hadley ME, Krajcarski DT, Szabo AG (1993) Structure activity correlations of melanotropic peptides in model lipids by tryptophan fluorescence studies. Biochemistry 32:12264–12272
Ito AS, Turchiello RF, Hirata IY, Cezari MH, Meldal M, Juliano L (1998) Fluorescent properties of amino acids labeled with orthoaminobenzoic. Biospectroscopy 4:395–402
Jiang Z, Vasil AI, Hale JD, Hancock RE, Vasil ML, Hodges RS (2008) Effects of net charge and the number of positively charged residues on the biological activity of amphipathic alpha-helical cationic antimicrobial peptides. Biopolymers (Pept Sci) 90:369–383
Kawato S, Kinosita K Jr, Ikegami A (1978) Effect of cholesterol on the molecular motion in the hydrocarbon region of lecithin bilayers studied by nanosecond fluorescence techniques. Biochemistry 17:5026–5031
Ladokhin AS (2001) On the interpretation of decay-associated fluorescence spectra in proteins. Biopolymers Cell 17:221–224
Ladokhin AS, Jayasinghe S, White SH (2000) How to measure and analyze the tryptophan fluorescence in membrane properly and why bother. Anal Biochem 285:235–245
Leite NB, da Costa LC, Alvares DS, Dos Santos Cabrera MP, De Souza BM, Palma MS, Ruggiero Neto J (2010) The effect of acidic residues and amphipathicity on the lytic activities of mastoparan peptides studied by fluorescence and CD spectroscopy. Amino Acids 40:91–100
Loura LMS, Ramalho JP (2007) Location and dynamics of acyl chain NBD-labeled phosphatidylcholine (NBD-PC) in DPPC bilayers. A molecular dynamics and time-resolved fluorescence anisotropy study. Biochim Biophys Acta 1768:467–478
Matsuzaki K (2009) Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta 1788:1687–1692
Moncrieffe MC, Juranic N, Kemple MD, Potter JD, Macura S, Prendergast FG (2000) Structure-fluorescence correlations in a single tryptophan mutant of carp parvalbumin: solution structure, backbone and side-chain dynamics. J Mol Biol 297:147–163
Pan C-P, Barkley MD (2004) Conformational effects on tryptophan fluorescence in cyclic hexapeptides. Biophys J 86:3828–3835
Raghuramanan H, Chattopadhyay A (2007) Orientation and dynamics of melittin in membranes of varying composition utilizing NBD fluorescence. Biophysical J 92:1271–1283
Rapson AC, Hossain MA, Wade JD, Nice EC, Smith TA, Clayton AHA, Gee ML (2011) Structural dynamics of a lytic peptide interacting with a supported lipid bilayer. Biophys J 100:1353–1361
Romani AP, Ito AS (2009) Interaction of adrenocorticotropin peptides with micro-heterogeneous systems. A fluorescence study. Biophys Chem 139:92–98
Romani AP, Marquezin CA, Soares AEE, Ito AS (2006) Study of the interaction between Apis mellifera venom and micro-heterogeneous systems. J Fluorescence 16:423–430
Romani AP, Marquezin CA, Ito AS (2010) Fluorescence spectroscopy of small peptides interacting with microheterogeneous micelles. Int J Pharmaceutics 383:154–156
Rouser G, Fleischer S, Yamamoto A (1970) Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5:494–496
Souza ES, Hirata IY, Juliano L, Ito AS (2000) End-to-end distribution distances in bradykinin observed by fluorescence energy transfer. Bioch Biophys Acta 1474:251–261
Takara M, Eisenhut JK, Hirata IY, Juliano L, Ito AS (2009) Solvent effects in optical spectra of ortho-aminobenzoic acid derivatives. J Fluoresc 19:1053–1060
Turchiello RF, Lamy-Freund MT, Hirata IY, Juliano L, Ito AS (1998) Ortho-aminobenzoic acid as a fluorescent probe for the interaction between peptides and micelles. Biophys Chem 73:217–225
Turchiello RF, Lamy-Freund MT, Hirata IY, Juliano Land Ito AS (2002) Ortho-aminobenzoic acid-labeled bradykinins in interaction with lipid vesicles: fluorescence study. Biopolymers 65:336–346
Wang KR, Zhang BZ, Zhang W, Yan JX, Li J, Wang R (2008) Antitumor effects and cell selectivity and structure-activity relationship of a novel antimicrobial peptide Polybia-MP1. Peptides 29:2320–2327
Wang K, Yan J, Zhang B, Song J, Jia J, Jia P, Wnag R (2009) Novel mode of action of polybia-MP1 a novel antimicrobial peptide in multidrug resistant leukemic cells. Cancer Lett 278:65–72
Wieprecht T, Dathe M, Schümann M, Krause E, Beyermann M, Bienert M (1996) Conformational and functional study of magainin 2 in model membrane environments using the new approach of systematic double-d-amino acid replacement. Biochemistry 35(33):10844–10853
Wimley WC, Hristova K (2011) Antimicrobial peptides: success, challenges and unanswered questions. J Membrane Biol 239:27–34
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Zhan H, Lazaridis T (2012) Influence of the membrane dipole potential on peptide binding to lipid bilayers. Biophys Chem 161:1–7
Zhao Kinnunen PJ (2002) Binding of the antimicrobial peptide Temporin L to liposomes assessed by trp fluorescence. J Biol Chem 277:25170–25177
Acknowledgments
JRN acknowledges the financial support from Sao Paulo Research Foundation (FAPESP), grant no. 2011/11640-5. ASI thanks the Brazilian agencies FAPESP, CNPq and INCT-FCx for financial support. Support was received from INCT. ASI and JRN are researchers at CNPq. LMPZ and WMP are recipients of a PhD grant from CAPES. DSA acknowledges the Sao Paulo Research Foundation (FAPESP) for PhD fellowship no. 2012/08147-8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zanin, L.M.P., dos Santos Alvares, D., Juliano, M.A. et al. Interaction of a synthetic antimicrobial peptide with model membrane by fluorescence spectroscopy. Eur Biophys J 42, 819–831 (2013). https://doi.org/10.1007/s00249-013-0930-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-013-0930-0