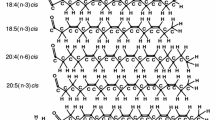
Abstract
Orientational order parameters and individual dihedral torsion angles are evaluated for phospholipid and glycolipid molecules that are resolved in X-ray structures of integral transmembrane proteins in crystals. The order parameters of the lipid chains and glycerol backbones in protein crystals are characterised by a much wider distribution of orientational order than is found in fluid lipid bilayers and reconstituted lipid–protein membranes. This indicates that the lipids that are resolved in crystals of membrane proteins are mostly not representative of the entire lipid–protein interface. Much of the chain configurational disorder of the membrane-bound lipids in crystals arises from C–C bonds in energetically disallowed skew conformations. This suggests configurational heterogeneity of the lipids at a single binding site: eclipsed conformations occur also in the glycerol backbone torsion angles and the C–C torsion angles of the lipid head groups. Conformations of the lipid glycerol backbone in protein crystals are not restricted to the gauche C1–C2 rotamers found invariably in phospholipid bilayer crystals. Lipid head-group conformations in the protein crystals also do not conform solely to the bent-down conformation, with gauche–gauche configuration of the phosphodiester, that is characteristic of phospholipid bilayer membranes. Stereochemical violations in the protein-bound lipids are evidenced by ester carboxyl groups in non-planar configurations, and even in the cis configuration. Some lipids have the incorrect enantiomeric configuration of the glycerol backbone, and many of the branched methyl groups in the phytanyl chains associated with bacteriorhodopsin have the incorrect S configuration.









Similar content being viewed by others
References
Abe A, Mark JE (1976) Conformational energies and the random-coil dimensions and dipole-moments of the polyoxides CH3O[(CH2) y O] x CH3. J Am Chem Soc 98:6468–6476
Abe A, Jernigan RL, Flory PJ (1966) Conformational energies of n-alkanes and the random configuration of higher homologs including polymethylene. J Am Chem Soc 88:631–650
Allinger NL, Zhu Z, Chen K (1992) Molecular mechanics (MM3) studies of carboxylic acids and esters. J Am Chem Soc 114:6120–6133
Belrhali H, Nollert P, Royant A, Menzel C, Rosenbusch JP, Landau EM, Pebay-Peyroula E (1999) Protein, lipid and water organization in bacteriorhodopsin crystals: a molecular view of the purple membrane at 1.9 Å resolution. Structure 7:909–917
Bertero MG, Rothery RA, Palak M, Hou C, Lim D, Blasco F, Weiner JH, Strynadka NCJ (2003) Insights into the respiratory electron transfer pathway from the structure of nitrate reductase. Nat Struct Biol 10:681–687
Bienvenue A, Bloom M, Davis JH, Devaux PF (1982) Evidence for protein-associated lipids from deuterium nuclear magnetic resonance studies of rhodopsin-dimyristoylphosphatidylcholine recombinants. J Biol Chem 257:3032–3038
Bloom M, Smith ICP (1985) Manifestations of lipid–protein interactions in deuterium NMR. In: Watts A, de Pont JJHHM (eds) Progress in protein–lipid interactions, vol 1. Elsevier, Amsterdam, pp 61–88
Borisova NP, Vol’kenshtein MV (1961) Internal rotation in propane and n-butane. Zh Strukt Khim 2:437–442
Camara-Artigas A, Brune D, Allen JP (2002) Interactions between lipids and bacterial reaction centers determined by protein crystallography. Proc Natl Acad Sci USA 99:11055–11060
Cevc G, Marsh D (1987) Phospholipid bilayers. Physical principles and models. Wiley–Interscience, New York
Chandrasekhar I, Kastenholz M, Lins RD, Oostenbrink C, Schuler LD, Tieleman DP, van Gunsteren WF (2003) A consistent potential energy parameter set for lipids: dipalmitoylphosphatidylcholine as a benchmark of the GROMOS96 45A3 force field. Eur Biophys J 32:67–77
de Planque MRR, Greathouse DV, Koeppe RE II, Schäfer H, Marsh D, Killian JA (1998) Influence of lipid/peptide hydrophobic mismatch on the thickness of diacylphosphatidylcholine bilayers. A 2H NMR and ESR study using designed transmembrane α-helical peptides and gramicidin A. Biochemistry 37:9333–9345
DePristo MA, de Bakker PIW, Blundell TL (2004) Heterogeneity and inaccuracy in protein structures solved by x-ray crystallography. Structure 12:831–838
East JM, Lee AG (1982) Lipid selectivity of the calcium and magnesium ion dependent adenosinetriphosphatase, studied with fluorescence quenching by a brominated phospholipid. Biochemistry 21:4144–4151
Elder M, Hitchcock P, Mason R, Shipley GG (1977) A refinement analysis of the crystallography of the phospholipid, 1,2-dilauroyl-dl-phosphatidylethanolamine, and some remarks on lipid–lipid and lipid–protein interactions. Proc R Soc Lond A 354:157–170
Esmann M, Powell GL, Marsh D (1988) Spin label studies on the selectivity of lipid–protein interaction of cardiolipin analogues with the Na+/K+-ATPase. Biochim Biophys Acta 941:287–292
Essen L-O, Siegert R, Lehmann WD, Oesterhelt D (1998) Lipid patches in membrane protein oligomers: crystal structure of the bacteriorhodopsin–lipid complex. Proc Natl Acad Sci USA 95:11673–11678
Ferguson AD, Welte W, Hofmann E, Lindner B, Holst O, Coulton JW, Diederichs K (2000) A conserved structural motif for lipopolysaccharide recognition by procaryotic and eucaryotic proteins. Struct Fold Des 8:585–592
Flory PJ (1969) Statistical mechanics of chain molecules. Wiley, London
Fyfe PK, Ridge JP, McAuley KE, Cogdell RJ, Isaacs NW, Jones MR (2000) Structural consequences of the replacement of glycine M203 with aspartic acid in the reaction center from Rhodobacter sphaeroides. Biochemistry 39:5953–5960
Fyfe PK, McAuley KE, Rothmann M, Isaacs NW, Cogdell RJ, Jones MR (2001) Probing the interface between membrane proteins and membrane lipids by x-ray crystallography. Trends Biochem Sci 26:106–112
Gally HU, Niederberger W, Seelig J (1975) Conformation and motion of the choline head group in bilayers of dipalmitoyl-3-sn-phosphatidylcholine. Biochemistry 14:3647–3652
Gorenstein DG, Kar D (1977) Effect of bond angle distortion on torsional potentials. Ab initio and CNDO/2 calculations on dimethoxymethane and dimethyl phosphate. J Am Chem Soc 99:672–677
Gorenstein DG, Kar D, Luxon BA, Momii RK (1976) Conformational study of cyclic and acyclic phosphate esters. CNDO/2 calculations of angle strain and torsional strain. J Am Chem Soc 98:1668–1673
Harlos K, Eibl H, Pascher I, Sundell S (1984) Conformation and packing properties of phosphatidic acid—the crystal structure of monosodium dimyristoylphosphatidate. Chem Phys Lipids 34:115–126
Harrenga A, Michel H (1999) The cytochrome c oxidase from Paracoccus denitrificans does not change the metal center ligation upon reduction. J Biol Chem 274:33296–33299
Hauser H, Pascher I, Pearson RH, Sundell S (1981) Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta 650:21–51
Hong M, Schmidt-Rohr K, Zimmermann H (1996) Conformational constraints on the headgroup and sn-2 chain of bilayer DMPC from NMR dipolar couplings. Biochemistry 35:8335–8341
Horváth LI, Brophy PJ, Marsh D (1988a) Exchange rates at the lipid–protein interface of myelin proteolipid protein studied by spin-label electron spin resonance. Biochemistry 27:46–52
Horváth LI, Brophy PJ, Marsh D (1988b) Influence of lipid headgroup on the specificity and exchange dynamics in lipid–protein interactions. A spin label study of myelin proteolipid apoprotein–phospholipid complexes. Biochemistry 27:5296–5304
Horváth LI, Brophy PJ, Marsh D (1990a) Influence of polar residue deletions on lipid–protein interactions with the myelin proteolipid protein. Spin-label ESR studies with DM-20/lipid recombinants. Biochemistry 29:2635–2638
Horváth LI, Drees M, Beyer K, Klingenberg M, Marsh D (1990b) Lipid–protein interactions in ADP–ATP carrier/egg phosphatidylcholine recombinants studied by spin-label ESR spectroscopy. Biochemistry 29:10664–10669
Horváth LI, Brophy PJ, Marsh D (1993) Exchange rates at the lipid–protein interface of the myelin proteolipid protein determined by saturation transfer electron spin resonance and continuous wave saturation studies. Biophys J 64:622–631
Horváth LI, Brophy PJ, Marsh D (1994) Microwave frequency dependence of ESR spectra from spin labels undergoing two-site exchange in myelin proteolipid membranes. J Magn Reson B105:120–128
IUPAC Commission on Nomenclature of Organic Chemistry (1976) Rules for the nomenclature of organic chemistry. Section E: stereochemistry. Pure Appl Chem 45:11–30
IUPAC-IUB Commission on Biochemical Nomenclature (CBN) (1970) Abbreviations and symbols for the description of the conformation of polypeptide chains. Eur J Biochem 17:193–201
IUPAC-IUB Commission on Biochemical Nomenclature (CBN) (1977) The nomenclature of lipids. Eur J Biochem 79:11–21
Joo CN, Kates M (1969) Synthesis of the naturally occurring phytanyl diether analogs of phosphatidyl glycerophosphate and phosphatidyl glycerol. Biochim Biophys Acta 176:278–297
Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauß N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 411:909–917
Jormakka M, Tornroth S, Byrne B, Iwata S (2002) Molecular basis of proton motive force generation: structure of formate dehydrogenase-N. Science 295:1863–1868
Kates M, Joo CN, Palameta B, Shier T (1967) Absolute stereochemical configuration of phytanyl (dihydrophytyl) groups in lipids of Halobacterium cutirubrum. Biochemistry 6:3329–3338
Kates M, Moldoveanu N, Stewart LC (1993) On the revised structure of the major phospholipid of Halobacterium salinarum. Biochim Biophys Acta 1169:46–53
King MD, Marsh D (1987) Headgroup and chain length dependence of phospholipid self-assembly studied by spin-label electron spin resonance. Biochemistry 26:1224–1231
Kleywegt GJ, Henrick K, Dodson EJ, van Aalten DMF (2003) Pound-wise but penny-foolish: how well do micromolecules fare in macromolecular refinement? Structure 11:1051–1059
Klyne W, Prelog V (1960) Description of steric relationships across single bonds. Experientia 16:521–523
Kurisu G, Zhang H, Smith JL, Cramer WA (2003) Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science 302:1009–1014
Lange C, Nett JH, Trumpower BL, Hunte C (2001) Specific roles of protein–phospholipid interactions in the yeast cytochrome bc 1 complex structure. EMBO J 20:6591–6600
Lee AG (2003) Lipid–protein interactions in biological membranes: a structural perspective. Biochim Biophys Acta 1612:1–40
Lee AG (2011) Biological membranes: the importance of molecular detail. Trends Biochem Sci 36:493–500
Li S, Lin HN, Wang ZQ, Huang C (1994) Identification and characterization of kink motifs in 1-palmitoyl-2-oleoyl-phosphatidylcholines: a molecular mechanics study. Biophys J 66:2005–2018
Liu ZF, Yan HC, Wang KB, Kuang TY, Zhang JP, Gui LL, An XM, Chang WR (2004) Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428:287–292
London E, Feigenson GW (1981) Fluorescence quenching in model membranes. 2. Determination of the local lipid environment of the calcium adenosine triphosphatase from sarcoplasmic reticulum. Biochemistry 20:1939–1948
Luecke H, Schobert B, Richter H-T, Cartailler J-P, Lanyi JK (1999) Structure of bacteriorhodopsin at 1.55 Å resolution. J Mol Biol 291:899–911
Marius P, Alvis SJ, East JM, Lee AG (2005) The interfacial lipid binding site on the potassium channel KcsA is specific for anionic phospholipids. Biophys J 89:4081–4089
Marius P, Zagnoni M, Sandison ME, East JM, Morgan H, Lee AG (2008) Binding of anionic lipids to at least three nonannular sites on the potassium channel KcsA is required for channel opening. Biophys J 94:1689–1698
Marsh D (1974) Statistical mechanics of the fluidity of phospholipid bilayers and membranes. J Membrane Biol 18:145–162
Marsh D (2003) Lipid-binding proteins: structure of the phospholipid ligands. Protein Sci 12:2109–2117
Marsh D (2008a) Electron spin resonance in membrane research: protein–lipid interactions. Methods 46:83–96
Marsh D (2008b) Energetics of hydrophobic matching in lipid–protein interactions. Biophys J 94:3996–4013
Marsh D, Horváth LI (1998) Structure, dynamics and composition of the lipid–protein interface. Perspectives from spin-labelling. Biochim Biophys Acta 1376:267–296
Marsh D, Páli T (2006) Lipid conformation in crystalline bilayers and in crystals of transmembrane proteins. Chem Phys Lipids 141:48–65
McAuley KE, Fyfe PK, Ridge JP, Isaacs NW, Cogdell RJ, Jones MR (1999) Structural details of an interaction between cardiolipin and an integral membrane protein. Proc Natl Acad Sci USA 96:14706–14711
Meier P, Sachse J-H, Brophy PJ, Marsh D, Kothe G (1987) Integral membrane proteins significantly decrease the molecular motion in lipid bilayers: a deuteron NMR relaxation study of membranes containing myelin proteolipid apoprotein. Proc Natl Acad Sci USA 84:3704–3708
Moser M, Marsh D, Meier P, Wassmer K-H, Kothe G (1989) Chain configuration and flexibility gradient in phospholipid membranes. Comparison between spin-label electron spin resonance and deuteron nuclear magnetic resonance, and identification of new conformations. Biophys J 55:111–123
Mouritsen OG, Bloom M (1984) Mattress model of lipid–protein interactions in membranes. Biophys J 46:141–153
Nogi T, Fathir I, Kobayashi M, Nozawa T, Miki K (2000) Crystal structures of photosynthetic reaction center and high-potential iron-sulfur protein from Thermochromatium tepidum: thermostability and electron transfer. Proc Natl Acad Sci USA 97:13561–13566
Oldfield E (1982) NMR of protein-lipid interactions in model and biological membrane systems. In: Martonosi EN (ed) Membranes and transport, vol 1. Plenum Press, New York, pp 115–123
Páli T, Marsh D (2001) Tilt, twist and coiling in β-barrel membrane proteins: relation to infrared dichroism. Biophys J 80:2789–2797
Pascher I (1996) The different conformations of the glycerol region of crystalline acylglycerols. Curr Opin Struct Biol 6:439–448
Pascher I, Sundell S (1986) Membrane lipids: preferred conformational states and their interplay. The crystal structure of dilauroylphosphatidyl-N,N-dimethylethanolamine. Biochim Biophys Acta 855:68–78
Pascher I, Sundell S, Harlos K, Eibl H (1987) Conformation and packing properties of membrane lipids: the crystal structure of sodium dimyristoylphosphatidylglycerol. Biochim Biophys Acta 896:77–88
Pascher I, Lundmark M, Nyholm P-G, Sundell S (1992) Crystal structures of membrane lipids. Biochim Biophys Acta 1113:339–373
Pearson RH, Pascher I (1979) The molecular structure of lecithin dihydrate. Nature (Lond) 281:499–501
Pebay-Peyroula E, Rosenbusch JP (2001) High-resolution structures and dynamics of membrane protein–lipid complexes: a critique. Curr Opin Struct Biol 11:427–432
Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trézéguet V, Lauquin GJM, Brandolin G (2003) Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426:39–44
Peelen SJCJ, Sanders JC, Hemminga MA, Marsh D (1992) Stoichiometry, selectivity, and exchange dynamics of lipid–protein interaction with bacteriophage M13 coat protein studied by spin label electron spin resonance. Effects of protein secondary structure. Biochemistry 31:2670–2677
Plesniak LA, Yu L, Dennis EA (1995) Conformation of micellar phospholipid bound to the active site of phospholipase A2. Biochemistry 34:4943–4951
Ponder JW, Richards FM (1987) Tertiary templates for proteins. Use of packing criteria in the enumeration of allowed sequences for different structural classes. J Mol Biol 193:775–791
Powell GL, Knowles PF, Marsh D (1985) Association of spin-labelled cardiolipin with dimyristoylphosphatidylcholine-substituted bovine heart cytochrome c oxidase. A generalized specificity increase rather than highly specific binding sites. Biochim Biophys Acta 816:191–194
Powell GL, Knowles PF, Marsh D (1987) Spin label studies on the specificity of interaction of cardiolipin with beef heart cytochrome oxidase. Biochemistry 26:8138–8145
Russell NJ, Harwood JL (1979) Changes in the acyl lipid composition of photosynthetic bacteria grown under photosynthetic and non-photosynthetic conditions. Biochem J 181:339–345
Ryba NJP, Marsh D (1992) Protein rotational diffusion and lipid/protein interactions in recombinants of bovine rhodopsin with saturated diacylphosphatidylcholines of different chain lengths studied by conventional and saturation transfer electron spin resonance. Biochemistry 31:7511–7518
Ryba NJP, Horváth LI, Watts A, Marsh D (1987) Molecular exchange at the lipid-rhodopsin interface: spin-label electron spin resonance studies of rhodopsin-dimyristoyl phosphatidylcholine recombinants. Biochemistry 26:3234–3240
Scott DL, Sigler PB (1994) Structure and catalytic mechanism of secretory phospholipase A2. Adv Protein Chem 45:53–88
Seelig J (1977) Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys 10:358–418
Seelig J, Gally HU (1976) Investigation of phosphatidylethanolamine bilayers by deuterium and phosphorus-31 nuclear magnetic resonance. Biochemistry 15:5199–5204
Seelig A, Seelig J (1974) Dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic resonance. Biochemistry 13:4839–4845
Seelig J, Seelig A (1980) Lipid conformation in model membranes and biological membranes. Q Rev Biophys 13:19–61
Seelig J, Gally HU, Wohlgemuth R (1977) Orientation and flexibility of the choline head group in phosphatidylcholine bilayers. Biochim Biophys Acta 467:109–119
Seelig J, Seelig A, Tamm L (1982) Nuclear magnetic resonance and lipid–protein interactions. In: Jost PC, Griffith OH (eds) Lipid–protein interactions, vol 2. Wiley, New York, pp 127–148
Stroebel D, Choquet Y, Popot JL, Picot D (2003) An atypical haem in the cytochrome b 6 f complex. Nature 426:413–418
Sundaralingam M (1972) Molecular structures and conformations of phospholipids and sphingomyelins. Ann N Y Acad Sci 195:324–355
Svensson-Ek M, Abramson J, Larsson G, Törnroth S, Brzezinski P, Iwata S (2002) The X-ray crystal structures of wild-type and EQ(I-286) mutant cytochrome c oxidases from Rhodobacter sphaeroides. J Mol Biol 321:329–339
Takeda K, Matsui Y, Sato H, Hino T, Kanamori E, Okumura H, Yamane T, Kamiya N, Kouyama T (2000) Deposition 1QM8 in the Protein Database
Thunnissen MMGM, Ab E, Kalk KH, Drenth J, Dijkstra BW, Kuipers OP, Dijkman R, de Haas GH, Verheij HM (1990) X-ray structure of phospholipase A2 complexed with a substrate derived inhibitor. Nature 347:689–691
Träuble H (1971) The movement of molecules across lipid membranes: a molecular theory. J Membrane Biol 4:193–208
Tsukihara T, Shimokata K, Katayama Y, Shimada H, Muramoto K, Aoyama H, Mochizuki M, Shinzawa-Itoh K, Yamashita A, Yao M, Ishimura Y, Yoshikawa S (2003) The low-spin heme of cytochrome c oxidase as the driving element of the proton-pumping process. Proc Natl Acad Sci USA 100:15304–15309
Valiyaveetil FI, Zhou YF, MacKinnon R (2002) Lipids in the structure, folding, and function of the KcsA K+ channel. Biochemistry 41:10771–10777
van Tilbeurgh H, Egloff MP, Martinez C, Rugani N, Verger R, Cambillau C (1993) Interfacial activation of the lipase–procolipase complex by mixed micelles revealed by X-ray crystallography. Nature 362:814–820
Yankovskaya V, Horsefield R, Törnröth S, Luna-Chavez C, Miyoshi H, Léger C, Byrne B, Cecchini G, Iwata S (2003) Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 299:700–704
Zhang Z, Huang L, Shulmeister VM, Chi Y-I, Kim KK, Hung L-W, Crofts AR, Berry EA, Kim S-H (1998) Electron transfer by domain movement in cytochrome bc 1. Nature 392:677–684
Acknowledgments
DM gratefully acknowledges financial assistance from Christian Griesinger and the Dept. of NMR-Based Structural Biology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: Structure, function, folding and assembly of membrane proteins—Insight from Biophysics.
Rights and permissions
About this article
Cite this article
Marsh, D., Páli, T. Orientation and conformation of lipids in crystals of transmembrane proteins. Eur Biophys J 42, 119–146 (2013). https://doi.org/10.1007/s00249-012-0816-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-012-0816-6