Abstract
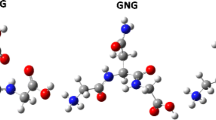
In many studies on the protein folding problem it is assumed that the internal rotational barriers about NCα and CαC backbone bonds in unfolded polypeptides are quite small, around 0.7 kcal/mol, of an order comparable to the energy of kT at normal temperature (where k is Boltzmann’s constant and T is the temperature in K) and hence that rotations about these bonds occur almost freely. Here it is highlighted that such consideration is an unfortunate mistake. Approximate values for the rotational barriers of NCα and CαC bonds are suggested from computations of U(\( \phi \), ψ) potential energy surface (PES) maps of a number of oligopeptides by a semiempirical method for conformational analysis. The proposed values are about 16 kcal/mol for NCα bonds and 6 kcal/mol for CαC bonds. The values of the same barriers estimated from some ab initio quantum-mechanical PES maps for several dipeptides available in literature are also highlighted.




Similar content being viewed by others
References
Baldwin RL (2007) Energetics of protein folding. J Mol Biol 371:283–301
Basharov MA (1995) Modified method of fragment–fragment interactions for studying intra- and intermolecular interactions. Russ J Phys Chem 69:1284–1288
Basharov MA, Vol’kenshtein MV, Golovanov IB, Ermakov GL, Nauchitel’ VV, Sobolev VM (1984a) Bond-bond interactions. A simple relationship for estimating the energy of a bond–bond interaction. J Struct Chem 25:26–29
Basharov MA, Vol’kenshtein MV, Golovanov IB, Sobolev VM (1984b) Bond-bond interactions. II. The barriers to internal rotations in saturated organic molecules. J Struct Chem 25:30–34
Basharov MA, Vol’kenshtein MV, Golovanov IB, Sobolev VM (1984c) Bond-bond interactions. III. Barriers to internal rotations in unsaturated hydrocarbons. J Struct Chem 25:173–177
Basharov MA, Vol’kenshtein MV, Golovanov IB, Ermakov GL, Sobolev VM (1984d) Bond-bond interactions. IV. Barriers to internal rotations in aldehydes, ketones, esters, organic acids, amides, and amino acids. J Struct Chem 25:177–181
Basharov MA, Vol’kenshtein MV, Golovanov IB, Ermakov GL, Nauchitel’ VV, Sobolev VM (1984e) Bond-bond interactions. V. Dimers of hydrogen molecules and hydrocarbons. J Struct Chem 25:356–360
Bhushan S, Gartman M, Halic M, Armache J-P, Jarasch A, Mielke T, Berninghausen O, Wilson DN, Beckman R (2010) α-Helical nascent polypeptide chains visualized within distinct region of the ribosomal exit tunnel. Nat Struct Mol Biol 17:313–317
Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus MJ (1983) CHARMM: a programm for macromolecular energy minimization, and dynamics calculations. Comput Chem 4:187–217
Cantor CR, Schimmel PR (1980) Biophysical chemistry. Part I. W.H. Freeman, San Francisco
Creighton TE (1990) Protein folding. Biochem J 270:1–16
Daggett V, Fersht AR (2003) The present view of the mechanism of protein folding. Nat Rev Mol Cell Biol 4:497–502
Ellis JR, Minton AP (2006) Protein aggregation in crowded environments. Biol Chem 387:485–497
Ellis RJ, Pinheiro TJT (2002) Danger—misfolding proteins. Nature 416:483–484
Engh RA, Huber R (1991) Accurate bond and angle parameters for X-ray protein structure refinement. Acta Cryst A47:392–400
Finkelstein AV, Ptitsyn OB (2002) Protein physics. Academic, London
Fitzkee NC, Rose GD (2004) Reassessing random-coil statistics in unfolded proteins. Proc Nat Acad Sci USA 101:12497–12502
Fitzkee NC, Fleming PJ, Gong H, Panasik N Jr, Street TO, Rose GD (2005) Are proteins made from a limited parts list? Trends Biochem Sci 30:73–80
Head-Gordon T, Head-Gordon M, Frisch MJ, Brooks CL III, Pople JA (1991) Theoretical study of blocked glycine and alanine peptide analogues. J Am Chem Soc 113:5989–5997
Hiller IH, Robson B (1979) Conformational behavior of the architectural units of peptides and proteins. Assessment of current understanding by ab initio quantum mechanical methods and refinement of the dipeptide energy surface. J Theor Biol 76:83–98
Lim V, Spirin AS (1986) Stereochemical analysis of ribosomal transpeptidation. J Mol Biol 188:565–574
McDonald DQ, Still WC (1992) AMBER torsional parameters for the peptide backbone. Tetrahedron Lett 33:7743–7746
Momany FA, McGuire RF, Burgess AW, Scheraga HA (1975) Energy parameters in polypeptides, VII: geometric parameters, partial atomic charges, nonbonded interactions, hydrogen bond interactions, and intrinsic torsional potentials for the naturally occurring amino acids. J Phys Chem 79:2361–2380
O’Connell TM, Wang L, Tropsha A, Hermans J (1999) The “random-coil” state of proteins: comparison of database statistics and molecular simulations. Prot: Struc Funct Genet 36:407–418
Peters D, Peters J (1981) Quantum theory of the structure and bonding in proteins. 8. The alanine dipeptide. J Mol Struct 85:107–123
Peters D, Peters J (1982a) Quantum theory of the structure and bonding in proteins. 13. The branched hydrocarbon side chains valine and isoleucine. J Mol Struct 88:157–170
Peters D, Peters J (1982b) Quantum theory of the structure and bonding in proteins. 14. The serine dipeptide. J Mol Struct 90:305–320
Peters D, Peters J (1982c) Quantum theory of the structure and bonding in proteins. 15. The threonine dipeptide. J Mol Struct 90:321–334
Ramachandran GN, Sasisekharan V (1968) Conformation of polypeptides and proteins. Adv Prot Chem 23:283–437
Scheraga HA (1968) Calculations of conformations of polypeptides. Adv Phys Org Chem 6:103–184
Smith LJ, Fiebig KM, Schwalbe H, Dobson CM (1996) The concept of a random coil. Residual structure in peptides and denatured proteins. Fold Des 1:R95–R106
Vasquez M, Nemethy G, Scheraga H (1994) Conformational energy calculations of polypeptides and proteins. Chem Rev 94:2183–2239
Weiner SJ, Kollman PA, Nguyen DT, Case DA (1986) An all atom force field for simulations of proteins and nucleic acids. J Comput Chem 7:230–252
Wright LR, Borkman RF (1982) Ab initio self-consistent field calculations on some small amino acids. J Phys Chem 86:3956–3962
Acknowledgments
I thank Lev Sarkisov for discussions. This work was funded by the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Basharov, M.A. The internal rotational barriers about NCα and CαC backbone bonds of polypeptides. Eur Biophys J 41, 53–61 (2012). https://doi.org/10.1007/s00249-011-0757-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-011-0757-5