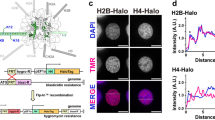
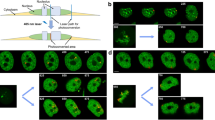
Abstract
Faithful chromatin segregation is mediated and controlled by the kinetochore protein network which assembles at centromeres. In this study, the neighbourhood relations of inner kinetochore and nucleosome-associated complex (NAC) proteins were analysed in living human interphase cells by acceptor photobleaching FRET. The data indicate that CENP-U is in close vicinity to CENP-I as well as to CENP-B and that CENP-M is close to CENP-T.





Similar content being viewed by others
References
Allshire RC, Karpen GH (2008) Epigentic regulation of centromeric chromatin: old dogs, new tricks? Nat Rev Gen 9:923–937
Ando S, Yang H, Nozaki N, Okazaki T, Yoda K (2002) Cenp-A, Cenp-B and Cenp-C chromatin complex that contains the I-type alpha-satellite array constitutes the prekinetochore in HeLa cells. Mol Cell Biol 22:2229–2241. doi:10.1128/MCB.22.7.2229-2241.2002
Berney C, Danuser G (2003) FRET or no FRET: a quantitative comparison. Biophys J 84:3992–4010. doi:10.1016/S0006-3495(03)75126-1
Black BE, Bassett EA (2008) The histone variant CENP-A and centromere specification. Cur Op Cell Biol 20:91–100
Black BE, Brock MA, Bedard S, Woods VL, Cleveland DW (2007a) An epigenetic mark generated by the incorporation of CENP-A into centromeric nucleosomes. Proc Natl Acad Sci USA 104:5008–5013
Black BE, Jansen LET, Maddox PS, Foltz DR, Desai AB, Shah JV, Cleveland DW (2007b) Centromere identity maintained by nucleosomes assembled with histone H3 containing the CENP-A targeting domain. Mol Cell 25:309–322
Carroll CW, Silva MCC, Godek KM, Jansen LET, Straight AF (2009) Centromere assembly requires the direct recognition of CENP-A nucleosomes by CENP-N. Nat Cell Biol (in press)
Cheeseman IM, Desai A (2008) Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol 9:33–46. doi:10.1038/nrm2310
Chen Y, Mills JD, Periasamy A (2003) Protein localisation in living cells and tissues using FRET and FLIM. Differentiation 71:528–541. doi:10.1111/j.1432-0436.2003.07109007.x
Cooke CA, Bernat RL, Earnshaw WC (1990) CENP-B: a major centromere protein located beneath the kinetochore. J Cell Biol 110:1475–1488. doi:10.1083/jcb.110.5.1475
Creemers TM, Lock AJ, Subramaniam V, Jovin TM, Völker S (1999) Three photoconvertible forms of green fluorescent protein identified by spectral hole-burning. Nat Struct Biol 6:557–560. doi:10.1038/10763
Elder AD, Domin A, Kaminski Schierle GS, Lindon C, Pines J, Esposito A, Kaminsiki CF (2009) A quantitative protocol for dynamic measurements of protein interactions by Förster resonance energy transfer-sensitized fluorescence emission. J R Soc Interface 6:S59–S81. doi:10.1098/rsif.2008.0381.focus
Foltz DR, Jansen ET, Black BE, Bailey AO, Yates III JR, Cleveland DW (2006) The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol 8:458–469. doi:10.1038/ncb1397
Hellwig D, Münch S, Orthaus S, Hoischen C, Hemmerich P, Diekmann S (2008) Live-cell imaging reveals sustained centromere binding of CENP-T via Cenp-A and Cenp-B. J Biophoton 1:245–254
Hemmerich P, Weidtkamp-Peters S, Hoischen C, Schmiedeberg L, Erliandri I, Diekmann S (2008) Dynamics of inner kinetochore assembly and maintenance in living cells. J Cell Biol 180:1101–1114. doi:10.1083/jcb.200710052
Hori T, Amano M, Suzuki A, Backer CB, Welburn JP, Dong Y, McEwan BF, Shang W-H, Suzuki E, Okawa K, Cheeseman IM, Fukagawa T (2008a) CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell 135:1039–1052. doi:10.1016/j.cell.2008.10.019
Hori T, Okada M, Maenaka K, Fukagawa T (2008b) CENP-O class proteins form a stable complex and are required for proper kinetochore function. Mol Biol Cell 19:843–854. doi:10.1091/mbc.E07-06-0556
Jares-Erijman EA, Jovin TM (2003) FRET imaging. Nat Biotechnol 21:1387–1395. doi:10.1038/nbt896
Jares-Erijman EA, Jovin TM (2006) Imaging molecular interactions in living cells by FRET microscopy. Curr Opin Chem Biol 10:409–416. doi:10.1016/j.cbpa.2006.08.021
Kenworthy AK (2001) Imaging protein–protein interactions using fluorescence resonance energy transfer microscopy. Methods 24:289–296. doi:10.1006/meth.2001.1189
Kenworthy AK, Edidin M (1998) Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 A using imaging fluorescence resonance energy transfer. J Cell Biol 142:69–84. doi:10.1083/jcb.142.1.69
Kitagawa K, Masumoto H, Ikeda M, Okazaki T (1995) Analysis of protein-DNA and protein-protein interactions of centromere protein B (CENP-B) and properties of the DNA-CENP-B complex in the cell cycle. Mol Cell Biol 15:1602–1612
Liu ST, Hittle JC, Jablonski SA, Campbell MS, Yoda K, Yen TJ (2003) Human CENP-I specifies localisation of CENP-F, MAD1 and MAD2 to kinetochores and is essential for mitosis. Nat Cell Biol 5:341–345. doi:10.1038/ncb953
Malvezzi-Campeggi F, Jahnz M, Heinze KG, Dittrich P, Schwille P (2001) Light-induced flickering of DsRed provides evidence for distinct and interconvertible fluorescent states. Biophys J 81:882–887
Marshall OW, Marshall AT, Choo KHA (2008) Three-dimensional localisation of CENP-A suggests a complex higher order structure of centromeric chromatin. J Cell Biol 183:1193–1202. doi:10.1083/jcb.200804078
McClelland SE, Borusu S, Amaro AC, Winter JR, Belwal M, McAinsh AD, Meraldi P (2007) The CENP-A NAC/CAD kinetochore complex controls chromosome congression and spindle bipolarity. EMBO J 26:5033–5047. doi:10.1038/sj.emboj.7601927
Minoshima Y, Hori T, Okada M, Kimura H, Haraguchi T, Hiraoka Y, Bao Y-C, Kawashima T, Kitamura T, Fukagawa T (2005) The constitutive centromere component CENP-50 is required for recovery from spindle damage. Mol Cell Biol 25:10315–10328. doi:10.1128/MCB.25.23.10315-10328.2005
Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8:379–393. doi:10.1038/nrm2163
Nagy P, Vamosi G, Bodnar A, Lockett SJ, Szöllösi J (1998) Intensity-based energy transfer measurements in digital imaging microscopy. Eur Biophys J 27:377–389. doi:10.1007/s002490050145
Nishihashi A, Haraguchi T, Hiraoka Y, Ikemura T, Regnier V, Dodson H, Earnshaw WC, Fukagawa T (2002) CENP-I is essential for centromere function in vertebrate cells. Dev Cell 2:463–476
Okada M, Cheeseman IM, Hori T, Okawa K, McLeod IX, Yates III JR, Desai A, Fukagawa T (2006) The CENP-H–I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat Cell Biol 8:446–457. doi:10.1038/ncb1396
Orthaus S, Biskup C, Hoffmann B, Hoischen C, Ohndorf S, Benndorf K, Diekmann S (2008) Assembly of the inner kinetochore proteins CENP-A and CENP-B in living human cells. ChemBioChem 9:77–92. doi:10.1002/cbic.200700358
Orthaus S, Klement K, Happel N, Hoischen C, Diekmann S (2009) Linker histone H1 is present in centromeric chromatin of living human cells next to inner kinetochore proteins. Nucl Acids Res. (Epub March 31) doi:10.1093/nar/gkp199
Patterson GH, Knobel SM, Sharif WD, Kain SR, Piston DW (1997) Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys J 73:2782–2790. doi:10.1016/S0006-3495(97)78307-3
Perpelescu M, Nozaki N, Obuse C, Yang H, Yoda K (2009) Active establishment of centromeric CENP-A chromatin by RSF complex. J Cell Biol 185:397–407. doi:10.1083/jcb.200903088
Pluta AF, Saitoh N, Goldberg I, Earnshaw WC (1992) Identification of a subdomain of CENP-B that is necessary and sufficient for localisation to the human centromere. J Cell Biol 116:1081–1093. doi:10.1083/jcb.116.5.1081
Roos UP (1973) Light and electron microscopy of rat kangaroo cells in mitosis. II. Kinetochore structure and function. Chromosoma 41:195–220. doi:10.1007/BF00319696
Sugimoto K, Kuriyama K, Shibata A, Himeno M (1997) Characterization of internal DNA-binding and C-terminal dimerization domains of human centromere/kinetochore autoantigen CENP-C in vitro: role of DNA-binding and self-associating activities in kinetochore organization. Chrom Res 5:132–141
Suzuki N, Nagano M, Nozaki N, Egashira S, Okazaki T, Masumoto H (2004) CENP-B interacts with CENP-C domains containing Mif2 regions responsible for centromere localization. J Biol Chem 279:5934–5946. doi:10.1074/jbc.M306477200
Tramier M, Zahid M, Mevel JC, Masse MJ, Coppey-Moisan M (2006) Sensitivity of CFP/YFP and GFP/mCherry pairs to donor photobleaching on FRET determination by fluorescence lifetime imaging microscopy in living cells. Microsc Res Tech 69:933–939. doi:10.1002/jemt.20370
Wieland G, Orthaus S, Ohndorf S, Diekmann S, Hemmerich P (2004) Functional complementation of human centromere protein A (CENP-A) by Cse4p from Saccharomyces cerevisiae. Mol Cell Biol 24:6620–6630. doi:10.1128/MCB.24.15.6620-6630.2004
Wouters FS, Bastiaens PIH, Wirtz KWA, Jovin TM (1998) FRET microscopy demonstrates molecular association of non-specific lipid transfer protein (nsL-TP) with fatty acid oxidation enzymes in peroxisomes. EMBO J 17:7179–7189. doi:10.1093/emboj/17.24.7179
Yoda K, Kitagawa K, Masumoto H, Muro Y, Okazaki T (1992) A human centromere protein, CENP-B, has a DNA binding domain containing four potential alpha helices at the NH2 terminus, which is separable from dimerising activity. J Cell Biol 119:1413–1427. doi:10.1083/jcb.119.6.1413
Acknowledgments
We thank N. Klöcker, D. Foltz, I. Cheeseman, M. Coppey-Moisan and N. Audugé for the kind gift of plasmids and the Deutsche Forschungs-Gemeinschaft (DFG) for support (SPP 1128).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article has been submitted as a contribution to the Festschrift entitled “Uncovering cellular sub-structures by light microscopy” in honour of Professor Cremer’s 65th birthday.
Rights and permissions
About this article
Cite this article
Hellwig, D., Hoischen, C., Ulbricht, T. et al. Acceptor-photobleaching FRET analysis of core kinetochore and NAC proteins in living human cells. Eur Biophys J 38, 781–791 (2009). https://doi.org/10.1007/s00249-009-0498-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-009-0498-x