Abstract
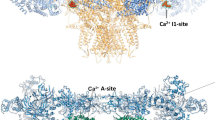
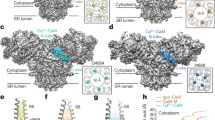
Contraction in skeletal and cardiac muscle occurs when Ca2+ is released from the sarcoplasmic reticulum (SR) through ryanodine receptor (RyR) Ca2+ release channels. Several isoforms of the RyR exist throughout the animal kingdom, which are modulated by ATP, Ca2+ and Mg2+ in the cytoplasm and by Ca2+ in the lumen of the SR. This review brings to light recent findings on their mechanisms of action in the mammalian isoforms RyR-1 and RyR-2 with an emphasis on RyR-1 from skeletal muscle. Cytoplasmic Mg2+ is a potent RyR antagonist that binds to two classes of cytoplasmic site, identified as low-affinity, non-specific inhibition sites and high-affinity Ca2+ activation sites (A-sites). Mg2+ inhibition at the A-sites is very sensitive to the cytoplasmic and luminal milieu. Cytoplasmic Ca2+, Mg2+ and monovalent cations compete for the A-sites. In isolated RyRs, luminal Ca2+ alters the Mg2+ affinity of the A-site by an allosteric mechanism mediated by luminal sites. However, in close-packed RyR arrays luminal Ca2+ can also compete with cytoplasmic ions for the A-site. Activation of RyRs by luminal Ca2+ has been attributed to either Ca2+ feedthrough to A-sites or to Ca2+ regulatory sites on the luminal side of the RyR. As yet there is no consensus on just how luminal Ca2+ alters RyR activation. Recent evidence indicates that both mechanisms operate and are likely to be important. Allosteric regulation of A-site Mg2+ affinity could trigger Ca2+ release, which is reinforced by Ca2+ feedthrough.




Similar content being viewed by others
References
Beard NA, Dulhunty AF, Laver DR (2000) The effect of increasing luminal calcium on skeletal muscle. Proceedings of the Australian Physiological and Pharmacological Society, vol 31, 22pp
Beard NA, Laver DR, Dulhunty AF (2004) Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol 85:33–69
Bhat MB, Zhao J, Takeshima H, Ma J (1997) Functional calcium release channel formed by the carboxyl-terminal portion of ryanodine receptor. Biophys J 73:1329–1336
Buck E, Zimanyi I, Abramson JJ, Pessah IN (1992) Ryanodine stabilizes multiple conformational states of the skeletal muscle calcium release channel. J Biol Chem 267:23560–23567
Ching LL, Williams AJ, Sitsapesan R (2000) Evidence for Ca2+ activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ Res 87:201–206
Chung SH, Moore JB, Xia LG, Premkumar LS, Gage PW (1990) Characterization of single channel currents using digital signal processing techniques based on Hidden Markov Models. Philos Trans R Soc Lond Biol 329:265–285
Copello JA, Porta M, Diaz-Silvester P, Nani A, Escobar AL, Fleischer S, Fill M (2003) Coordinated gating of multiple ryanodine receptor channels (RyRs). Biophys J 84:17a
Coronado R, Morrissette J, Sukhareva M, Vaughan DM (1994) Structure and function of ryanodine receptors. Am J Physiol 266:C1485–C1504
Donoso P, Prieto H, Hidalgo C (1995) Luminal calcium regulates calcium release in triads isolated from frog and rabbit skeletal muscle. Biophys J 68:507–515
Du GG, Guo X, Khanna VK, MacLennan DH (2001) Ryanodine sensitizes the cardiac Ca2+ release channel (ryanodine receptor isoform 2) to Ca2+ activation and dissociates as the channel is closed by Ca2+ depletion. Proc Natl Acad Sci USA 98:13625–13630
Dunnett J, Nayler WG (1978) Calcium efflux from cardiac sarcoplasmic reticulum: effects of calcium and magnesium. J Mol Cell Cardiol 10:487–498
Dutka TL, Lamb GD (2004) Effect of low cytoplasmic [ATP] on excitation–contraction coupling in fast-twitch muscle fibres of the rat. J Physiol 560:451–468
Endo M (1985) Calcium release from sarcoplasmic reticulum. Curr Topics Membr Trans 25:181–230
Fabiato A, Fabiato F (1977) Calcium release from the sarcoplasmic reticulum. Circ Res 40:119–129
Fleischer S, Ogunbunmi EM, Dixon MC, Fleer EA (1985) Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci USA 82:7256–7259
Ford LE, Podolsky RJ (1972) Calcium uptake and force development by skinned muscle fibres in EGTA buffered solutions. J Physiol (Lond) 223:1–19
Franzini-Armstrong C (1970) Studies of the triad. I. Structure of the junction in frog twitch fibres. J Cell Biol 47:488–498
Gilchrist JS, Belcastro AN, Katz S (1992) Intraluminal Ca2+ dependence of Ca2+ and ryanodine-mediated regulation of skeletal muscle sarcoplasmic reticulum Ca2+ release. J Biol Chem 267:20850–20856
Godt RE, Maughan DW (1988) On the composition of the cytosol of relaxed skeletal muscle of the frog. Am J Physiol 254:C591–C604
Gyorke I, Gyorke S (1998) Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J 75:2801–2810
Gyorke I, Hester N, Jones LR, Gyorke S (2004) The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J 86:2121–2128
Haarmann CS, Dulhunty AF, Laver DR (2005) Regulation of skeletal ryanodine receptors by dihydropyridine receptor II–III loop C-region peptides: relief of Mg2+ -inhibition. Biochem J 387(Pt 2):429–436
Hillyard IW, Procita L (1959) The effect of ryanodine on the contractile strength of mammalian cardiac (atrial) muscle. J Pharmacol Exp Ther 127:22–28
Humerickhouse RA, Besch HR Jr, Gerzon K, Ruest L, Sutko JL, Emmick JT (1993) Differential activating and deactivating effects of natural ryanodine congeners on the calcium release channel of sarcoplasmic reticulum: evidence for separation of effects at functionally distinct sites. Mol Pharmacol 44:412–421
Inui M, Saito A, Fleischer S (1987) Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. 262:1740–1747
Jenden DJ, Fairhurst AS (1969) The pharmacology of ryanodine. Pharmacol Rev 21:1–25
Jona I, Szegedi C, Sarkozi S, Szentesi P, Csernoch L, Kovacs L (2001) Altered inhibition of the rat skeletal ryanodine receptor/calcium release channel by magnesium in the presence of ATP. Pflugers Arch 441:729–738
Kermode H, Williams AJ, Sitsapesan R (1998) The interactions of ATP, ADP, and inorganic phosphate with the sheep cardiac ryanodine receptor. Biophys J 74:1296–1304
Lai FA, Anderson K, Rousseau E, Lui QY, Meissner G (1988) Evidence for a Ca2+ channel within the ryanodine receptor complex from cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun 151:441–449
Lai FA, Misra M, Xu L, Smith HA, Meissner G (1989) The ryanodine receptor-Ca2+ release channel complex of skeletal muscle sarcoplasmic reticulum. Evidence for a cooperatively coupled, negatively charged homotetramer. J Biol Chem 264:16776–16785
Lamb GD (2002) Voltage-sensor control of Ca2+ release in skeletal muscle: insights from skinned fibers. Front Biosci 7:d834–d842
Lamb GD, Stephenson DG (1991) Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. J Physiol (Lond) 434:507–528
Lamb GD, Stephenson DG (1992) Importance of Mg2+ in excitation–contraction coupling in skeletal muscle. News Physiol Sci 7:270–274
Lamb GD, Stephenson DG (1994) Effects of intracellular pH and [Mg2+] on excitation–contraction coupling in skeletal muscle fibres of the rat. J Physiol (Lond) 478:331–339
Lamb GD, Cellini MA, Stephenson DG (2001) Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J Physiol (Lond) 531:715–728
Lattanzio FA Jr, Schlatterer RG, Nicar M, Campbell KP, Sutko JL (1987) The effects of ryanodine on passive calcium fluxes across sarcoplasmic reticulum membranes. J Biol Chem 262:2711–2718
Laver D (2001) The power of single channel recording and analysis: its application to ryanodine receptors in lipid bilayers. Clin Exp Pharmacol Physiol 28:675–686
Laver DR, Gage PW (1997) Interpretation of substates in ion channels: unipores or multipores? Prog Biophys Mol Biol 67:99–140
Laver DR, Roden LD, Ahern GP, Eager KR, Junankar PR, Dulhunty AF (1995) Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J Membr Biol 147:7–22
Laver DR, Baynes TM, Dulhunty AF (1997) Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. J Membr Biol 156:213–229
Laver DR, Eager KR, Taoube L, Lamb GD (2000) Effects of cytoplasmic and luminal pH on Ca2+ release channels from rabbit skeletal muscle. Biophys J 78:1835–1851
Laver DR, Lenz GK, Lamb GD (2001) Regulation of the calcium release channel from rabbit skeletal muscle by the nucleotides ATP, AMP, IMP and adenosine. J Physiol (Lond) 537:763–778
Laver DR, O’Neill ER, Lamb GD (2004) Luminal Ca2+ regulated Mg2+ -inhibition of skeletal RyRs reconstituted as isolated channels or coupled clusters. J Gen Physiol 124:741–758
Ma J, Zhao J (1994) Highly cooperative and hysteretic response of the skeletal muscle ryanodine receptor to changes in proton concentrations. Biophys J 67:626–633
Marx SO, Ondrias K, Marks AR (1998) Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors). Science 281:818–821
Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR (2001) Coupled gating between cardiac calcium release channels (ryanodine receptors). Circ Res 88:1151–1158
Masumiya H, Li P, Zhang L, Chen SR (2001) Ryanodine sensitizes the Ca2+ release channel (ryanodine receptor) to Ca2+ activation. J Biol Chem 276:39727–39735
Meissner G (1984) Adenine nucleotide stimulation of Ca2+ -induced Ca2+ release in sarcoplasmic reticulum. J Biol Chem 259:2365–2374
Meissner G (1986) Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem 261:6300–6306
Meissner G (1994) Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Ann Rev Physiol 56:485–508
Meissner G, Darling E, Eveleth J (1986) Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry 25:236–244
Meissner G, Rousseau E, Lai FA, Liu QY, Anderson KA (1988) Biochemical characterization of the Ca2+ release channel of skeletal and cardiac sarcoplasmic reticulum. Mol Cell Biochem 82:59–65
Meissner G, Rios E, Tripathy A, Pasek DA (1997) Regulation of skeletal muscle Ca2+ release channel (ryanodine receptor) by Ca2+ and monovalent cations and anions. J Biol Chem 272:1628–1638
Melzer W, Herrmann-Frank A, Luttgau HC (1995) The role of Ca2+ ions in excitation–contraction coupling of skeletal muscle fibres. Biochim Biophys Acta 1241:59–116
Morii H, Tonomura Y (1983) The gating behavior of a channel for Ca2+ -induced Ca2+ release in fragmented sarcoplasmic reticulum. J Biochem (Tokyo) 93:1271–1285
Ogawa Y (1994) Role of ryanodine receptors. Crit Rev Biochem Mol Biol 29:229–274
Ondrias K, Mojzisova A (2002) Coupled gating between individual cardiac ryanodine calcium release channels. Gen Physiol Biophys 21:73–84
O’Neill ER, Sakowska MM, Laver DR (2003) Regulation of the calcium release channel from skeletal muscle by suramin and the disulfonated stilbene derivatives DIDS, DBDS, and DNDS. Biophys J 84:1674–1689
Owen VJ, Taske NL, Lamb GD (1997) Reduced inhibitory effect of Mg2+ on Ca2+ release in porcine muscle fibers with ryanodine receptor mutation for malignant hyperthermia. Am J Physiol 272:C203–C211
Pessah IN, Francini AO, Scales DJ, Waterhouse AL, Casida JE (1986) Calcium-ryanodine receptor complex. Solubilization and partial characterization from skeletal muscle junctional sarcoplasmic reticulum vesicles. J Biol Chem 261:8643–8648
Porta M, Diaz-Sylvester PN, Nani A, Fill M, Fleischer S, Copello JA (2004) Modulation of coordinated gating of ryanodine receptor (RyR) channels in planar lipid bilayers. Biophys J 86:241a
Posterino GS, Lamb GD, Stephenson DG (2000) Twitch and tetanic force responses and longitudinal propagation of action potentials in skinned skeletal muscle fibres of the rat. J Physiol (Lond) 527:131–137
Procita L (1956) The action of ryanodine on mammalian skeletal muscle in situ. J Pharmacol Exp Ther 117:363–373
Protasi F, Franzini-Armstrong C, Flucher BE (1997) Coordinated incorporation of skeletal muscle dihydropyridine receptors and ryanodine receptors in peripheral couplings of BC3H1 cells. J Cell Biol 137:859–870
Rousseau E, Smith JS, Meissner G (1987) Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol 253:C364–C368
Rousseau E, Pinkos J, Savaria D (1992) Functional sensitivity of the native skeletal Ca2+ -release channel to divalent cations and the Mg-ATP complex. Can J Physiol Pharmacol 70:394–402
Samso M, Wagenknecht T (1998) Contributions of electron microscopy and single-particle techniques to the determination of the ryanodine receptor three-dimensional structure. J Struct Biol 121:172–180
Sitsapesan R, Williams AJ (1994) Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca2+ -release channel by luminal Ca2+ . J Membr Biol 137:215–226
Sitsapesan R, Williams AJ (1995) The gating of the sheep skeletal sarcoplasmic reticulum Ca2+ -release channel is regulated by luminal Ca2+ . J Membr Biol 146:133–144
Sitsapesan R, Williams AJ (1997) Regulation of current flow through ryanodine receptors by luminal Ca2+. J Membr Biol 159:179–185
Smith JS, Coronado R, Meissner G (1986) Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+ . J Gen Physiol 88:573–588
Smith JS, Imagawa T, Ma J, Fill M, Campbell KP, Coronado R (1988) Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol 92:1–26
Soler F, Fernandez Belda F, Gomez Fernandez JC (1992) The Ca2+ release channel in junctional sarcoplasmic reticulum: gating and blockade by cations. Int J Biochem 24:903–909
Stern MD (1992) Buffering of calcium in the vicinity of a channel pore. Cell Calcium 13:183–192
Sutko JL, Airey JA (1996) Ryanodine receptor Ca2+ release channels: does diversity in form equal diversity in function? Physiol Rev 76:1027–1071
Tanabe T, Beam KG, Adams BA, Niidome T, Numa S (1990) Regions of the skeletal muscle dihydropyridine receptor critical for excitation–contraction coupling. Nature 346:567–569
Tripathy A, Meissner G (1996) Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys J 70:2600–2615
Xu L, Meissner G (1998) Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+ . Biophys J 75:2302–2312
Xu L, Meissner G (2004) Mechanism of calmodulin inhibition of cardiac sarcoplasmic reticulum Ca2+ release channel (ryanodine receptor). Biophys J 86:797–804
Acknowledgements
Thanks to G.D. Lamb for reading and commenting on the manuscript. This work was supported by the National Health and Medical Research Council of Australia (grant number 234420) and by an infrastructure grant from NSW Health through Hunter Medical Research Institute. This work was supported by a Professorial Fellowship from the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laver, D.R. Coupled calcium release channels and their regulation by luminal and cytosolic ions. Eur Biophys J 34, 359–368 (2005). https://doi.org/10.1007/s00249-005-0483-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-005-0483-y