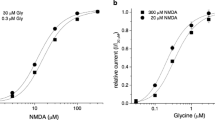
Abstract
To analyze the influence of the β-subunit on the kinetic properties of GlyR channel currents, α1-subunits and α1β-subunits were transiently expressed in HEK 293 cells. A piezo dimorph was used for fast application of glycine to outside-out patches. The rise time of activation was dose dependent for both receptors and decreased with increasing glycine concentrations. Subunit composition had no effect on the time course of activation. Coexpression of α1- and β-subunits resulted in a significantly lower EC50 and a reduced slope of the dose–response curve of glycine compared with expression of α1-subunits alone. For both receptor subtypes, the time course of desensitization was concentration dependent. Desensitization was best fitted with a single time constant at 10–30 µM, with two at 0.1 mM, and at saturating concentrations (0.3–3 mM) with three time constants. Desensitization of homomeric α1-receptor channels was significantly slower than that of α1β-receptor channels. The time course of current decay after the end of glycine pulses was tested at different pulse durations of 1 mM glycine. It was best fitted with two time constants for both α1 and α1β GlyR channels, and increased significantly with increasing pulse duration.






Similar content being viewed by others
References
Becker CM, Hoch W, Betz H (1988) Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J 7:3717–3726
Betz H (1990) Ligand-gated ion channels in the brain: the amino acid receptor superfamily. Neuron 5:383–392
Betz H, Kuhse J, Schmieden V, Malosio ML, Langosch D, Prior P, Schmitt B, Kirsch J (1991) How to build a glycinergic postsynaptic membrane. J Cell Sci Suppl 15:23–25
Bormann J, Rundstrom N, Betz H, Langosch D (1993) Residues within transmembrane segment M2 determine chloride conductance of glycine receptor homo- and hetero-oligomers. EMBO J 12:3729–3737
Bufler J, Franke C, Parnas H, Dudel J (1996) Open channel block by physostigmine and procaine in embryonic-like nicotinic receptors of mouse muscle. Eur J Neurosci 8:677–687
Franke C, Hatt H, Dudel J (1987) Liquid filament switch for ultra-fast exchanges of solutions at excised patches of synaptic membrane of crayfish muscle. Neurosci Lett 77:199–204
Graham D, Pfeiffer F, Simler R, Betz H (1985) Purification and characterization of the glycine receptor of pig spinal cord. Biochemistry 24:990–994
Grenningloh G, Schmieden V, Schofield PR, Seeburg PH, Siddique T, Mohandas TK, Becker CM, Betz H (1990) Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J 9:771–776
Grewer C (1999) Investigation of the alpha(1)-glycine receptor channel-opening kinetics in the submillisecond time domain. Biophys J 77:727–738
Haas KF, Macdonald RL (1999) GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol 514:27–45
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391:85–100
Handford CA, Lynch JW, Baker E, Webb GC, Ford JH, Sutherland GR, Schofield PR (1996) The human glycine receptor beta subunit: primary structure, functional characterisation and chromosomal localisation of the human and murine genes. Brain Res Mol Brain Res 35:211–219
Harty TP, Manis PB (1998) Kinetic analysis of glycine receptor currents in ventral cochlear nucleus. J Neurophysiol 79:1891–1901.
Jahn K, Bufler J, Franke C (1998) Kinetics of AMPA-type glutamate receptor channels in rat caudate-putamen neurones show a wide range of desensitization but distinct recovery characteristics. Eur J Neurosci 10:664–672
Jahn K, Mohammadi B, Krampfl K, Abicht A, Lochmuller H, Bufler J (2001) Deactivation and desensitization of mouse embryonic- and adult-type nicotinic receptor channel currents. Neurosci Lett 307:89–92
Jones MV, Westbrook GL (1995) Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron 15:181–191
Kandel ER, Schwartz JH, Jesell TM (1995) Essentials of neural science and behaviour. Appleton & Lange, Stanford, Conn.
Krampfl K, Bufler J, Lepier A, Dudel J, Adelsberger H (2000) Desensitization characteristics of rat recombinant GABA(A) receptors consisting of alpha1beta2gamma2S and alpha1beta2 subunits expressed in HEK293 cells. Neurosci Lett 278:21–24
Krampfl K, Schlesinger F, Zorner A, Kappler M, Dengler R, Bufler J (2002a) Control of kinetic properties of GluR2 flop AMPA-type channels: impact of R/G nuclear editing. Eur J Neurosci 15:51–62
Krampfl K, Wolfes H, Dengler R, Bufler J (2002b) Kinetic analysis of the agonistic and blocking properties of pentobarbital on recombinant rat alpha(1)beta(2)gamma(2S) GABA(A) receptor channels. Eur J Pharmacol 435:1–8
Kuhse J, Schmieden V, Betz H (1990) A single amino acid exchange alters the pharmacology of neonatal rat glycine receptor subunit. Neuron 5:867–873
Kuhse J, Kuryatov A, Maulet Y, Malosio ML, Schmieden V, Betz H (1991) Alternative splicing generates two isoforms of the alpha 2 subunit of the inhibitory glycine receptor. FEBS Lett 283:73–77
Langosch D, Becker CM, Betz H (1990) The inhibitory glycine receptor: a ligand-gated chloride channel of the central nervous system. Eur J Biochem 194:1–8
Langosch D, Laube B, Rundstrom N, Schmieden V, Bormann J, Betz H (1994) Decreased agonist affinity and chloride conductance of mutant glycine receptors associated with human hereditary hyperekplexia. EMBO J 13:4223–4228
Laube B, Kuhse J, Betz H (2000) Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol 577:215–230
Legendre P (1997) Pharmacological evidence for two types of postsynaptic glycinergic receptors on the Mauthner cell of 52-h-old zebrafish larvae. J Neurophysiol 77:2400–2415
Legendre P (1998) A reluctant gating mode of glycine receptor channels determines the time course of inhibitory miniature synaptic events in zebrafish hindbrain neurons. J Neurosci 18:2856–2870
Lewis TM, Sivilotti LG, Colquhoun D, Gardiner RM, Schoepfer R, Rees M (1998) Properties of human glycine receptors containing the hyperekplexia mutation alpha1(K276E), expressed in Xenopus oocytes. J Physiol 507:25–40
Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H (1991) Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J 10:2401–2409
Mohammadi B, Krampfl K, Moschref H, Dengler R, Bufler J (2001) Interaction of the neuroprotective drug riluzole with GABA(A) and glycine receptor channels. Eur J Pharmacol 415:135–140
Rajendra S, Lynch JW, Pierce KD, French CR, Barry PH, Schofield PR (1995) Mutation of an arginine residue in the human glycine receptor transforms beta-alanine and taurine from agonists into competitive antagonists. Neuron 14:169–175
Schmieden V, Grenningloh G, Schofield PR, Betz H (1989) Functional expression in Xenopus oocytes of the strychnine binding 48 kd subunit of the glycine receptor. EMBO J 8:695–700
Schmieden V, Kuhse J, Betz H (1992) Agonist pharmacology of neonatal and adult glycine receptor alpha subunits: identification of amino acid residues involved in taurine activation. EMBO J 11:2025–2032
Singer JH, Berger AJ (1999) Contribution of single-channel properties to the time course and amplitude variance of quantal glycine currents recorded in rat motoneurons. J Neurophysiol 81:1608–1616.
Twyman RE, Macdonald RL (1991) Kinetic properties of the glycine receptor main- and sub-conductance states of mouse spinal cord neurones in culture. J Physiol 435:303–331
Unwin N (1989) The structure of ion channels in membranes of excitable cells. Neuron 3:665–676
Watanabe E, Akagi H (1995) Distribution patterns of mRNAs encoding glycine receptor channels in the developing rat spinal cord. Neurosci Res 23:377–382
Wyllie DJA, Béhé P, Colquhoun D (1998) Single-channel activation and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol 610:1–18
Acknowledgements
We thank Prof. H. Betz, Frankfurt, for the kind gift of cDNAs of glycine receptors, U. Jensen for expert technical assistance, and A. Niesel and J. Kilian for technical support. This study was supported by grants from the Deutsche Forschungsgemeinschaft and the Medizinische Hochschule Hannover.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammadi, B., Krampfl, K., Cetinkaya, C. et al. Kinetic analysis of recombinant mammalian α1 and α1β glycine receptor channels. Eur Biophys J 32, 529–536 (2003). https://doi.org/10.1007/s00249-003-0286-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-003-0286-y