Abstract
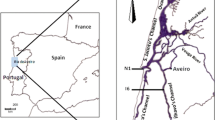
The possibility that two principlal bacterial communities expressing different levels of heterotrophic activity might coexist in an estuarine ecosystem (Ria de Aveiro, Portugal) and could quickly respond to tidal fluctuations of environmental factors was experimentally tested in diffusion chambers by swapping the dissolved components of the natural water between the two communities and comparing their reactivity against the unaltered controls. The results for ectoenzymatic activity (Leuaminopeptidase and β-glucosidase), glucose incorporation and biomass production after transference of the marine bacterial community to brackish water showed maxima in the range of 241–384% of the control values. The opposite transference of the brackish-water bacterial community to marine water produced maximal decreases to 0.14–0.58% of the control values. In a reverse experiment, designed as the return to the initial conditions after 2 hours of the first exposure, the marine community rapidly re-acquired the characteristic low profile of activity. Contrastingly, the negative effects of 2 hours of exposure to marine water on the activity of the brackish water bacteria persisted, at least for 4 hours, after return to their own water. The apparent short-term irreversibility of the decline in activity of the brackish water bacteria when exposed to marine water, in parallel with the quick and reversible positive response of the marine water bacteria to the brackish water, suggests the development of two distinct bacterioplankton communities adapted to the environmental conditions prevailing at distinct sections of the estuary. The reactivity to environmental changes demonstrated by the two communities allows the prediction of estuarine profiles of bacterial activity steeper than those expected from the conservative transport of bacterial cells associated with tidal currents.
Similar content being viewed by others
References
Abreu S Duarte A (1997) Biogest First Cientific Report—Environment and Climate Programme. Contract N. ENV4-CT96-0213 (Partner 6 participation).
American Public Health Association (1992) Standard Methods for the Examination of Water and Waste Water., pp
Barillier A, Garnier J (1993) Influence of temperature and substrate concentration on bacterial growth yield in Seine River water batch cultures. Appl Environ Microbiol 59:1678–1682
Biddanda B, Opshal S, Benner R (1994) Plankton respiration and carbon flux through bacterioplankton on the Louisiana Shelf. Limnol Oceanogr 39:1259–1275
Bordalo AA, Pinto MM, Carvalho LM (1988) Dinâmica sazonal e espacial de variáveis abióticas e bióticas no estuário do Rio Douro. Actas do 1o Simpósio Interdisciplinar sobre Processos Estuatinos, Universidade do Algarve, Faro, pp 47–48
Christian RR, Stanley DW, Daniel DA (1984) Microbiological changes occurring at the freshwater-seawater interface of the Neuse River Estuary, North Carolina. In: Kennedy VS (ed) The Estuary as a Filter. Academic Press, Orlando, pp 349–365
Chróst RJ (1991) Environmental control of the synthesis and activity of aquatic microbial ectoenzymes. In: Chróst RJ (ed) Microbial Enzymes in Aquatic Environments. Springer-Verlag, New York, pp 29–59
Crottereau C, Delmas D (1988) Exoproteolytic activity in an Atlantic pond (France): estimates of in situ activity. Aquat Microb Ecol 15:217–224
Cunha MA, Almeida MA, Alcântara F (1999) Compartments of oxygen consumption in a tidal mesotrophic estuary (Ria de Aveiro, Portugal). Acta Oecologica 20:227–235
Cunha MA, Almeida MA (2000) Patterns of ectoenzymatic and heterotrophic bacterial activities alng a salinity gradient in a shallow tidal estuary. Mar Ecol Prog Ser 204:1–12
Eppley RW, Harrison WG, Chisholm SW, Stewart E (1977) Particulate organic matter in surface waters off Southern California and its relationships to phytoplankton. J Mar Res 35:671–696
Fuks D, Devescovi M, Precali R, Krstulovic N, Solic M (1991) Bacterial abundance and activity in the highly stratified estuary of the Krka River. Mar Chem 32:333–346
Gocke K (1977) Comparison of methods for determining the turnover times of dissolved organic compounds. Mar Biol 42:131–141
Griffith PC, Douglas DJ, Wainright SC (1990) Metabolic activity of size-fractionated microbial plankton in estuarine, nearshore and continental shelf waters of Georgia. Mar Ecol Prog Ser 59:263–270
Hobbie JE, Daley R, Jasper S (1977) Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33:1225–1228
Hopkinson C, Giblin AE, Garritt RH, Tucker J, Hullar MAJ (1998) Influence of benthos on growth of planktonic estuarine bacteria. Aquat Microb Ecol 16:109–118
Hoppe HG (1983) Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methyllumbeliferyl-substrates. Mar Ecol Prog Ser 11:299–308
Hoppe HG, Giesenhagen HC, Gocke K (1998) Changing patterns of bacterial substrate decomposition in a eutrophication gradient. Aquat Microb Ecol 15:1–13
Jellett JF, Li WKW, Dickie PM, Boraie A, Kepkay PE (1996) Metabolic activity of bacterioplankton communities assessed by flow cytometry and single carbon substrate utilization. Mar Ecol Prog Ser 136:213–225
Jørgensen NOG, Kroer N, Coffin RB, Hoch MP (1999) Relations between bacterial nitrogen metabolism and growth efficiency in an estuarine and in an open-water ecosystem. Aquat Microb Ecol 18:247–261
Karner M, Fuks D, Herndl GJ (1992) Bacterial activity along atrophic gradient. Microb Ecol 24:243–257
Martinez J, Smith DC, Steward GF, Azam F (1996) Variability in ecotohydrolytic enzyme activities of pelagic marine bacteria and its significance for substrate processing in the sea. Aquat Microb Ecol 10:223–230
Middelboe M, Kroer N, Jørgensen NOG, Pakulski D (1998) Influence of sediment on pelagic carbon and nitrogen turn-over in a shallow Danish estuary. Aquat Microb Ecol 14:81–90
Murrell MC, Hollibaugh JT, Solver MW, Wong PS (1999) Bacterioplankton dynamics in northern San Francisco Bay: Role of particle association and seasonal freshwater flow. Limnol Oceanogr 44:295–308
Palumbo A, Ferguson RL (1978) Distribution of suspended bacteria in the Newport River estuary, North Carolina. Estuar Coast Mar Sci 7:251–259
Palumbo AV, Fergusson RL, Rublee PA (1984) Size of suspended bacterial cells and association of heterotrophic activity with size fractions of particles in estuarine and coastal waters. Appl Environ Microbiol 48:157–164
Parsons TR, Maita Y, Lalli CM (1989) A Manual of Chemical and Biological Methods for Seawater Analysis. Pergamon Press, Oxford, pp
Pereira MG, Alcântara F (1993) Culturability of Escherichia coli and Streptococcus faecalis in batch culture and in situ in estuarine water (Portugal). Wat Res 27:1351–1360
Pinhassi J, Azam F, Hemphälä J, Long RA, Martinez J, Zweifel UL, Hagström Å (1999) Coupling between bacterioplankton species composition, population dynamics and organic matter degradation. Aquat Microb Ecol 17:13–26
Rodier J (1996) L'analyse de l'eau: eaux naturelles, eaux résidaaires, eau de mer, 8th ed. Dunod, Paris, pp
Sañudo-Wilhelmy SA, Taylor GT (1999) Bacterioplankton dynamics and organic carbon partitioning in the lower River Hudson estuary. Mar Ecol Prog Ser 182:17–27
Shiah FK, Ducklow HW (1995) Multiscale variability in bacterioplankton abundance, production and specific growth rate in a temperate salt-marsh tidal creek. Limnol Oceanogr 40:55–66
Silva JJF, Circulação de água na ria de Aveiro: contribuição para o estudo da qualidade da água. PhD thesis, Universidade de Aveiro (Portugal). 1994
Simon M, Azam F (1989) Protein content and protein synthesis rates of planktonic bacteria. Mar Ecol Prog Ser 51:201–213
Treguer P, Le Corre P (1975) Manuel d'analyse des sels nutritifs dans l'eau de mer. Labotatorie d'Oceanologie Chimique, Université de Bretagne Occidentale, France, pp
Unanue M, Ayo B, Agis M, Slezak D, Herndl GJ, Iriberri J (1999) Ectoenzymatic activity and uptake of monomers in marine bacterioplankton described by a biphasic kinetic model. Microb Ecol 37:36–48
Wikner J, Cuadros R, Jansson M (1999) Differences in consumption of allochthonous DOC under limnic and estuarine conditions. Aquat Microb Ecol 17:289–299
Wright RT, Coffin RB (1983) Planktonic bacteria in estuaries and coastal waters off Northern Massachusetts: spatial and temporal distribution. Mar Ecol Prog Ser 11:205–216
Yentsch CS, Menzel DW (1963) A method for the determination of phytoplankton chlorophyll and phaeophytin by fluorescence. Deep-Sea Res 10:221–231
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cunha, M.A., Almeida, M.A. & Alcântara, F. Short-term responses of the natural planktonic bacterial community to the changing water properties in an estuarine environment: Ectoenzymatic activity, glucose incorporation, and biomass production. Microb Ecol 42, 69–79 (2001). https://doi.org/10.1007/s002480000098
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002480000098