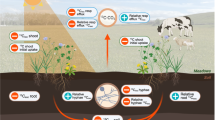
Abstract
Rain events in arid environments are highly unpredictable and intersperse extended periods of drought. Therefore, tracking changes in desert soil bacterial communities during rain events, in the field, was seldom attempted. Here, we assessed rain-mediated dynamics of active bacterial communities in the Negev Desert biological soil crust (biocrust). Biocrust samples were collected during, and after a medium rainfall and dry soil was used as a control; we evaluated the changes in active bacterial composition, potential function, potential photosynthetic activity, and extracellular polysaccharide (EPS) production. We hypothesized that rain would activate the biocrust phototrophs (mainly Cyanobacteria), while desiccation would inhibit their activity. In contrast, the biocrust Actinobacteria would decline during rewetting and revive with desiccation. Our results showed that hydration increased chlorophyll content and EPS production. As expected, biocrust rewetting activated Cyanobacteria, which replaced the former dominant Actinobacteria, boosting potential autotrophic functions. However, desiccation of the biocrust did not immediately change the bacterial composition or potential function and was followed by a delayed decrease in chlorophyll and EPS levels. This dramatic shift in the community upon rewetting led to modifications in ecosystem services. We propose that following a rain event, the response of the active bacterial community lagged behind the biocrust water content due to the production of EPS which delayed desiccation and temporarily sustained the biocrust community activity.





Similar content being viewed by others
Data Availability
The data (raw reads) are available in Bioproject under the submission number PRJNA718159.
References
Aanderud ZT, Bahr J, Robinson DM, Belnap J, Campbell TP, Gill RA, McMillian B, St. Clair S (2019) The burning of biocrusts facilitates the emergence of a bare soil community of poorly-connected chemoheterotrophic bacteria with depressed ecosystem services. Front Ecol Evol 7:1–14. https://doi.org/10.3389/fevo.2019.00467
Agarwal L, Qureshi A, Kalia VC, Kapley A, Purohit HJ, Singh RN (2014) Arid ecosystem: future option for carbon sinks using microbial community intelligence. Curr Sci 106:1357–1363
Angel R (2012) Total nucleic acid extraction from soil. Research Square - Protocols, 1–5. https://doi.org/10.1038/protex.2012.046
Angel R, Conrad R (2013) Elucidating the microbial resuscitation cascade in biological soil crusts following a simulated rain event. Environ Microbiol 15:2799–2815. https://doi.org/10.1111/1462-2920.12140
Angel R, Pasternak Z, Soares MIM, Conrad R, Gillor O (2013) Active and total prokaryotic communities in dryland soils. FEMS Microbiol Ecol 86:130–138. https://doi.org/10.1111/1574-6941.12155
Bache SM, Wickham H, Henry L (2014) Magrittr: a forward-pipe operator for R. R Package Version 2.0.3
Barbera P, Kozlov AM, Czech L, Morel B, Darriba D, Flouri T, Stamatakis A (2019) EPA-ng: massively parallel evolutionary placement of genetic sequences. Syst Biol. https://doi.org/10.1093/sysbio/syy054
Barnard RL, Osborne CA, Firestone MK (2013) Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J 7:2229–2241. https://doi.org/10.1038/ismej.2013.104
Baubin C, Farrell AM, Šťovíček A, Ghazaryan L, Giladi I, Gillor O (2019) Seasonal and spatial variability in total and active bacterial communities from desert soil. Pedobiologia (Jena) 74:7–14. https://doi.org/10.1016/J.PEDOBI.2019.02.001
Bay SK, McGeoch MA, Gillor O, Wieler N, Palmer DJ, Baker DJ, Chown SL, Greening C (2020) Soil bacterial communities exhibit strong biogeographic patterns at fine taxonomic resolution. mSystems 5:1–16. https://doi.org/10.1128/msystems.00540-20
Belnap J (1999) Structure and function of biological soil crusts. In: Sagebrush steppe ecosystems symposium, pp 55–62
Belnap J, Lange OL (2001) Biological soil crusts: structure, function, and management. Springer. https://doi.org/10.1639/0007-2745(2002)105[0500:]2.0.co;2
Blazewicz SJ, Barnard RL, Daly RA, Firestone MK (2013) Evaluating rRNA as an indicator of microbial activity in environmental communities: limitations and uses. ISME J 7:2061–2068. https://doi.org/10.1038/ismej.2013.102
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu Y-X, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37(8):852–857. https://doi.org/10.1038/s41587-019-0209-9
Borisov VB, Forte E, Davletshin A, Mastronicola D, Sarti P, Giuffrè A (2013) Cytochrome bd oxidase from Escherichia coli displays high catalase activity: an additional defense against oxidative stress. FEBS Lett 587:2214–2218. https://doi.org/10.1016/j.febslet.2013.05.047
Bowker MA, Reed SC, Maestre FT, Eldridge DJ (2018) Biocrusts: the living skin of the earth. Plant Soil 429:1–7. https://doi.org/10.1007/s11104-018-3735-1
Bruins HJ (2012) Ancient desert agriculture in the Negev and climate-zone boundary changes during average, wet and drought years. J Arid Environ 86:28–42. https://doi.org/10.1016/j.jaridenv.2012.01.015
Büdel B, Darienko T, Deutschewitz K, Dojani S, Friedl T, Mohr KI, Salisch M, Reisser W, Weber B (2009) Southern african biological soil crusts are ubiquitous and highly diverse in drylands, being restricted by rainfall frequency. Microb Ecol 57:229–247. https://doi.org/10.1007/s00248-008-9449-9
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13(7):581–583. https://doi.org/10.1038/nmeth.3869
Cameron RE, Blank GB (1966) Desert algae: soil crusts and diaphanous substrata as algal habitats. National Aeronautics and Space Administration, Pasadena, California
Campbell SE, Seeler J, Golubic S (1989) Desert crust formation and soil stabilization. Arid Soil Res Rehabil 3:217–228. https://doi.org/10.1080/15324988909381200
Carter MR, Gregorich EG (2008) Soil sampling and methods of analysis (Second Edition), Canadian Society of Soil Science. CRC Press. https://doi.org/10.1017/s0014479708006546
Castillo-Monroy AP, Maestre FT, Rey A, Soliveres S, Garcia-Palacios P (2011) Biological soil crust microsites are the main contributor to soil respiration in a semiarid ecosystem. Ecosystems 14:835–847. https://doi.org/10.1007/s10021-011-9449-3
Castle SC, Morrison CD, Barger NN (2010) Extraction of chlorophyll a from biological soil crusts: a comparison of solvents for spectrophotometric determination. Soil Biol Biochem 43:853–856. https://doi.org/10.1016/j.soilbio.2010.11.025
Colica G, Li H, Rossi F, Li D, Liu Y, de Philippis R (2014) Microbial secreted exopolysaccharides affect the hydrological behavior of induced biological soil crusts in desert sandy soils. Soil Biol Biochem 68:62–70. https://doi.org/10.1016/j.soilbio.2013.09.017
Cordero PRF, Bayly K, Man Leung P, Huang C, Islam ZF, Schittenhelm RB, King GM, Greening C (2019) Atmospheric carbon monoxide oxidation is a widespread mechanism supporting microbial survival. ISME J 13:2868–2881. https://doi.org/10.1038/s41396-019-0479-8
Couradeau E, Giraldo-Silva A, de Martini F, Garcia-Pichel F (2019) Spatial segregation of the biological soil crust microbiome around its foundational cyanobacterium, Microcoleus vaginatus, and the formation of a nitrogen-fixing cyanosphere. Microbiome 7:1–12. https://doi.org/10.1186/s40168-019-0661-2
Czech L, Barbera P, Stamatakis A (2020) Genesis and Gappa: Processing, analyzing and visualizing phylogenetic (placement) data. Bioinformatics 36:3263–3265. https://doi.org/10.1093/bioinformatics/btaa070
Dechesne A, Wang G, Gülez G, Or D, Smets BF (2010) Hydration-controlled bacterial motility and dispersal on surfaces. Proc Natl Acad Sci U S A 107:14369–14372. https://doi.org/10.1073/pnas.1008392107
Dinno A (2017) dunn.test: Dunn’s test of multiple comparisons using rank sums. R Package Version 1.3.5
Douglas GM, Maffel VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, Huttenhower C, Langille MG (2020) PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. https://doi.org/10.1038/s41587-020-0550-z
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://pubs.acs.org/sharingguidelines
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6:241–252
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vörösmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Goodfellow M, Williams ST (1983) Ecology of actinomycetes. Annu Rev Microbiol 37:189–216. https://doi.org/10.1146/annurev.mi.37.100183.001201
Greening C, Biswas A, Carere CR, Jackson CJ, Taylor MC, Stott MB, Cook GM, Morales SE (2016) Genomic and metagenomic surveys of hydrogenase distribution indicate H 2 is a widely utilised energy source for microbial growth and survival. ISME J 10:761–777. https://doi.org/10.1038/ismej.2015.153
Halverson LJ, Jones TM, Firestone MK (2000) Release of intracellular solutes by four soil bacteria exposed to dilution stress. Soil Sci Soc Am J 64:1630–1637. https://doi.org/10.2136/sssaj2000.6451630x
Hansen BB, Henriksen S, Aanes R, Sæther BE (2007) Ungulate impact on vegetation in a two-level trophic system. Polar Biol 30:549–558. https://doi.org/10.1007/s00300-006-0212-8
Harris RF (1981) Effect of water potential on microbial growth and activity. In: Water potential relations in soil microbiology, pp 23–95. https://doi.org/10.2136/sssaspecpub9.c2
Henrikus SS, Wood EA, McDonald JP, Cox MM, Woodgate R, Goodman MF, van Oijen AM, Robinson A (2018) DNA polymerase IV primarily operates outside of DNA replication forks in Escherichia coli. PLoS Genet 14:1–29. https://doi.org/10.1371/journal.pgen.1007161
Hill AJ, Lincoln NK, Rachmilevitch S, Shelef O (2020) Modified hiltner dew balance to re-estimate dewfall accumulation as a reliable water source in the Negev Desert. Water (Switzerland) 12(10):1–10. https://doi.org/10.3390/w12102952
Hoogsteen MJJ, Lantinga EA, Bakker EJ, Groot JCJ, Tittonell PA (2015) Estimating soil organic carbon through loss on ignition: effects of ignition conditions and structural water loss. Eur J Soil Sci 66:320–328. https://doi.org/10.1111/ejss.12224
Kaneshisa M, Goto S (2000) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28:27–30. https://doi.org/10.3892/ol.2020.11439
Karaoz U, Couradeau E, da Rocha UN, Lim HC, Northen T, Garcia-Pichel F, Brodie EL (2018) Large blooms of Bacillales (Firmicutes) underlie the response to wetting of cyanobacterial biocrusts at various stages of maturity. mBio 9:1–17. https://doi.org/10.1128/mBio.01366-16
Kedem I, Treves H, Noble G, Hagemann M, Murik O, Raanan H, Oren N, Giordano M, Kaplan A (2020) Keep your friends close and your competitors closer: novel interspecies interaction in desert biological sand crusts. Phycologia 00:1–8. https://doi.org/10.1080/00318884.2020.1843349
Kidron GJ (1999) Altitude dependent dew and fog in the Negev Desert, Israel. Agric For Meteorol 96:1–8
Kidron GJ, Tal SY (2012) The effect of biocrusts on evaporation from sand dunes in the Negev Desert. Geoderma 179–180:104–112. https://doi.org/10.1016/j.geoderma.2012.02.021
Kidron GJ, Herrnstadt I, Barzilay E (2002) The role of dew as a moisture source for sand microbiotic crusts in the Negev Desert, Israel. J Arid Environ 52:517–533. https://doi.org/10.1006/jare.2002.1014
Kidron GJ, Li XR, Jia RL, Gao YH, Zhang P (2015) Assessment of carbon gains from biocrusts inhabiting a dunefield in the Negev Desert. Geoderma 253–254:102–110. https://doi.org/10.1016/j.geoderma.2015.04.015
Kidron GJ, Wang Y, Herzberg M (2020) Exopolysaccharides may increase biocrust rigidity and induce runoff generation. J Hydrol 588:125081. https://doi.org/10.1016/j.jhydrol.2020.125081
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47:583–621. https://doi.org/10.1080/01621459.1952.10483441
Lan S, Wu L, Zhang D, Hu C (2012) Successional stages of biological soil crusts and their microstructure variability in Shapotou region (China). Environmental Earth Sciences 65:77–88. https://doi.org/10.1007/s12665-011-1066-0
Lennon JT, Jones SE (2011) Microbial seed banks: the ecological and evolutionary implications of dormancy. Nat Rev Micro 9:119–130
León-Sobrino C, Ramond JB, Maggs-Kölling G, Cowan DA (2019) Nutrient acquisition, rather than stress response over diel cycles, drives microbial transcription in a hyper-arid Namib desert soil. Front Microbiol 10:1–11. https://doi.org/10.3389/fmicb.2019.01054
Leung PM, Bay SK, Meier DV, Chiri E, Cowan DA, Gillor O, Woebken D, Greening C (2020) Energetic basis of microbial growth and persistence in desert ecosystems. mSystems 5:1–14. https://doi.org/10.1128/msystems.00495-19
Louca S, Doebeli M (2018) Efficient comparative phylogenetics on large trees. Bioinformatics 34:1053–1055. https://doi.org/10.1093/bioinformatics/btx701
Mager DM, Thomas AD (2011) Extracellular polysaccharides from cyanobacterial soil crusts: a review of their role in dryland soil processes. J Arid Environ 75:91–97. https://doi.org/10.1016/j.jaridenv.2010.10.001
Malek E, McCurdy G, Giles B (1999) Dew contribution to the annual water balances in semi-arid desert valleys. J Arid Environ 42:71–80. https://doi.org/10.1006/jare.1999.0506
Mazor G, Kidron GJ, Vonshak A, Abeliovich A (1996) The role of cyanobacterial exopolysaccharides in structuring desert microbial crusts. FEMS Microbiol Ecol 21:121–130. https://doi.org/10.1016/0168-6496(96)00050-5
McMurdie PJ, Holmes S (2013) Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8(4). https://doi.org/10.1371/JOURNAL.PONE.0061217
Meier DV, Imminger S, Gillor O, Woebken D (2021) Distribution of mixotrophy and desiccation survival mechanisms across microbial genomes in an arid biological soil crust community. mSystems 6:1–20. https://doi.org/10.1128/msystems.00786-20
Murik O, Oren N, Shotland Y, Raanan H, Treves H, Kedem I, Keren N, Hagemann M, Pade N, Kaplan A (2017) What distinguishes cyanobacteria able to revive after desiccation from those that cannot: the genome aspect. Environ Microbiol 19:535–550. https://doi.org/10.1111/1462-2920.13486
Murrell P (2018) Grid: a rewrite of the graphics layout capabilities, plus some support for interaction. R Package Version 4.3.0. https://doi.org/10.1201/b10966-6
Noy-Meir I (1973) Desert ecosystems: environment and producers. Annu Rev Ecol Syst 4:25–52. https://doi.org/10.1146/annurev.es.04.110173.000325
Nunes da Rocha U, Cadillo-Quiroz H, Karaoz U, Rajeev L, Klitgord N, Dunn S, Truong V, Buenrostro M, Bowen BP, Garcia-Pichel F, Mukhopadhyay A, Northen TR, Brodie EL (2015) Isolation of a significant fraction of non-phototroph diversity from a desert biological soil crust. Front Microbiol 6:277. https://doi.org/10.3389/fmicb.2015.00277
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2014) Vegan: community ecology package. R package version 2.5-1
Oren N, Raanan H, Murik O, Keren N, Kaplan A (2017) Dawn illumination prepares desert cyanobacteria for dehydration. Curr Biol 27:R1056–R1057. https://doi.org/10.1016/j.cub.2017.08.027
Oren N, Raanan H, Kedem I, Turjeman A, Bronstein M, Kaplan A, Murik O (2019) Desert cyanobacteria prepare in advance for dehydration and rewetting: the role of light and temperature sensing. Mol Ecol 28:2305–2320. https://doi.org/10.1111/mec.15074
Péli ER, Lei N, Pócs T, Laufer Z, Porembski S, Tuba Z (2011) Ecophysiological responses of desiccation-tolerant cryptobiotic crusts. Cent Eur J Biol 6:838–849. https://doi.org/10.2478/s11535-011-0049-1
Pointing SB, Belnap J (2012) Microbial colonization and controls in dryland systems. Nat Rev Microbiol 10:551–562. https://doi.org/10.1038/nrmicro2831
Preiss J (1984) Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol 38:419–458
Preiss J, Sivak M (1999) 3.14 - Starch and glycogen biosynthesis. In: Barton SD, Nakanishi K, Meth-Cohn OBT-CNPC (eds). Pergamon, Oxford, pp 441–495. https://doi.org/10.1016/B978-0-08-091283-7.00082-5
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41(D1):D590–D596. https://doi.org/10.1093/nar/gks1219
Rajeev L, da Rocha UN, Klitgord N, Luning EG, Fortney J, Axen SD, Shih PM, Bouskill NJ, Bowen BP, Kerfeld CA, Garcia-Pichel F, Brodie EL, Northen TR, Mukhopadhyay A (2013) Dynamic cyanobacterial response to hydration and dehydration in a desert biological soil crust. ISME J 7:2178–2191. https://doi.org/10.1038/ismej.2013.83
Repar J, Briski N, Buljubašić M, Zahradka K, Zahradka D (2012) Exonuclease VII is involved in “reckless” DNA degradation in UV-irradiated Escherichia coli. Mutat Res 750:96–104. https://doi.org/10.1016/j.mrgentox.2012.10.005
Ritchie RJ (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89:27–41. https://doi.org/10.1007/s11120-006-9065-9
Roberson EB, Firestone MK (1992) Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl Environ Microbiol 58:1284–1291. https://doi.org/10.1128/aem.58.4.1284-1291.1992
Román JR, Roncero-Ramos B, Rodríguez-Caballero E, Chamizo S, Cantón Y (2021) Effect of water availability on induced cyanobacterial biocrust development. Catena (Amst) 197:104988. https://doi.org/10.1016/j.catena.2020.104988
Shelef O, Groner E (2011) Linking landscape and species: effect of shrubs on patch preference of beetles in arid and semi-arid ecosystems. J Arid Environ 75(10):960–967. https://doi.org/10.1016/j.jaridenv.2011.04.016
Sklarz MY, Levin L, Gordon M, Chalifa-Caspi V (2018) NeatSeq-Flow: a lightweight high throughput sequencing workflow platform for non-programmers and programmers alike. bioRxiv 173005. https://doi.org/10.1101/173005
Slade D, Radman M (2011) Oxidative stress resistance in Deinococcus radiodurans. Microbiol Mol Biol Rev 75:133–191. https://doi.org/10.1128/mmbr.00015-10
Steven B, Belnap J, Kuske CR (2018) Chronic physical disturbance substantially alters the response of biological soil crusts to a wetting pulse, as characterized by metatranscriptomic sequencing. Front Microbiol 9:2382. https://doi.org/10.3389/fmicb.2018.02382
Št’ovíček A, Kim M, Or D, Gillor O (2017) Microbial community response to hydration-desiccation cycles in desert soil. Sci Rep 7:45735. https://doi.org/10.1038/srep45735
Sukenik A, Kaplan-Levy RN, Welch JM, Post AF (2012) Massive multiplication of genome and ribosomes in dormant cells (akinetes) of Aphanizomenon ovalisporum (Cyanobacteria). ISME J 6:670–679. https://doi.org/10.1038/ismej.2011.128
Sunyer-Figueres M, Wang C, Mas A (2018) Analysis of ribosomal RNA stability in dead cells of wine yeast by quantitative PCR. Int J Food Microbiol 270:1–4. https://doi.org/10.1016/j.ijfoodmicro.2018.01.020
Tveit AT, Hestnes AG, Robinson SL, Schintlmeister A, Dedysh SN, Jehmlich N, von Bergen M, Herbold C, Wagner M, Richter A, Svenning MM (2019) Widespread soil bacterium that oxidizes atmospheric methane. Proc Natl Acad Sci U S A 116:8515–8524. https://doi.org/10.1073/pnas.1817812116
van Goethem MW, Swenson TL, Trubl G, Roux S, Northen TR (2019) Characteristics of wetting-induced bacteriophage blooms in biological soil crust. mBio 10. https://doi.org/10.1128/mBio.02287-19
Veste M, Littmann T, Friedrich H, Breckle SW (2001) Microclimatic boundary conditions for activity of soil lichen crusts in sand dunes of the north-western Negev desert, Israel. Flora 196:465–474. https://doi.org/10.1016/S0367-2530(17)30088-9
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer New York. https://doi.org/10.1007/978-0-387-98141-3
Wickham H (2017) Scales: scale functions for visualization. R Package Version 1.0.0. https://cran.r-project.org/web/packages/scales/scales.pdf
Wickham H, Francois R, Henry L, Müller K (2018) dplyr: a grammar of data manipulation. R Package Version 0.7.8
Xu H-F, Raanan H, Dai G-Z, Oren N, Berkowicz S, Murik O, Kaplan A, Qiu B-S (2021) Reading and surviving the harsh conditions in desert biological soil crust: the cyanobacterial viewpoint. FEMS Microbiol Rev 45:fuab036. https://doi.org/10.1093/femsre/fuab036
Ye Y, Doak TG (2009) A parsimony approach to biological pathway reconstruction/inference for genomes and metagenomes. PLoS Comput Biol 5:e1000465. https://doi.org/10.1371/JOURNAL.PCBI.1000465
Zangvil A (1996) Six years of dew observations in the Negev Desert, Israel. J Arid Environ 32:361–371
Zvyagintsev DG, Zenova GM, Doroshenko EA, Gryadunova AA, Gracheva TA, Sudnitsyn II (2007) Actinomycete growth in conditions of low moisture. Biology Bulletin 34:242–247. https://doi.org/10.1134/S1062359007030053
Acknowledgements
The authors are grateful to Lusine Ghazaryan for technical support and to Ben Poodiack and Kate Kaufman for editing the manuscript.
Funding
This study was partially supported by the Israel Science Academy, grant no. 993/11.
Author information
Authors and Affiliations
Contributions
CB, OG, and HS conceptualized and designed the methodology; CB and OG collected the samples and metadata; CB and NR did the laboratory work and sequencing; CB did the formal analysis, visualization, data curation, and wrote the manuscript; CB, OG, HS, and NR did the reviewing and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baubin, C., Ran, N., Siebner, H. et al. Divergence of Biocrust Active Bacterial Communities in the Negev Desert During a Hydration-Desiccation Cycle. Microb Ecol 86, 474–484 (2023). https://doi.org/10.1007/s00248-022-02063-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-022-02063-z