Abstract
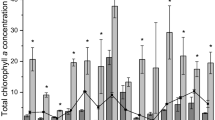
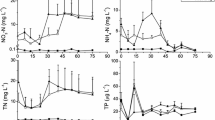
The ecological importance of phytoplankton-benthic-propagules (PBP) from coastal sediments, except tropical monsoon-influenced rivers and estuaries, is well documented. PBP in such systems is often transported from upstream (near freshwater) to downstream (estuary) through freshwater discharges during monsoon and thereby experiences higher salinities (>30 PSU) and nutrients with varying light conditions due to reducing discharges during the monsoon-break/withdrawal-phase. However, the PBP responses (germination and subsequent growth) to such changes are unknown and are examined here at ~35 PSU salinity. For the study, macronutrients (nitrate, phosphate, silicate, and nitrate+phosphate+silicate) and light intensities were considered to assess the response of PBP representing estuarine, brackish, and near freshwater locations of monsoon-influenced Mandovi and Zuari rivers (Goa, India). Diatoms dominated the viable PBP community, but the maximum growth and sustained photosynthetic activity were observed when all macronutrients were supplied than individually. Here, variable fluorescence technique utility in PBP resurrection (detection of viability and responses) was also explored. The PBP growth was similar for macronutrients but increased with light intensity indicating a longer growth response during monsoon. For PBP (germination and photosynthetic activity), light intensities drive the rate of improvement/development, whereas the nutrients are essential for maintaining vegetative growth upon germination in the region. The PBP dominance of common planktonic species (Skeletonema and Thalassiosira) along the river also signifies the role of seawater intrusion (up to upstream locations) in distribution. Skeletonema and Thalassiosira, which contribute significantly to the total community, are light-sensitive with a similar response and cause single species blooms during monsoon and non-monsoon, respectively, depending on the species’ tolerance to salinity.








Similar content being viewed by others
Data Availability
Please contact the author for data requests.
References
Not F, Siano R, Kooistra WH, Simon N, Vaulot D, Probert I (2012) Diversity and ecology of eukaryotic marine phytoplankton. Adv Bot Res 64:1–53. https://doi.org/10.1016/B978-0-12-391499-6.00001-3
Figueroa RI, Estrada M, Garcés E (2018) Life histories of microalgal species causing harmful blooms: Haploids, diploids and the relevance of benthic stages. Harmful Algae 73:44–57. https://doi.org/10.1016/j.hal.2018.01.006
Azanza RV, Brosnahan ML, Anderson DM, Hense I, Montresor M (2018) The role of life cycle characteristics in harmful algal bloom dynamics. In: Glibert P, Berdalet E, Burford M, Pitcher G, Zhou M. (eds) Global Ecology and Oceanography of Harmful Algal Blooms. Ecological Studies (Analysis and Synthesis), vol 232. Springer, Cham. https://doi.org/10.1007/978-3-319-70069-4_8
Hallegraeff GM, Bolch C (1992) Transport of diatom and dinoflagellate resting spores in ships’ ballast water: implications for plankton biogeography and aquaculture. J Plank Res 14(8):1067–1084
Godhe A, Härnström K (2010) Linking the planktonic and benthic habitat: genetic structure of the marine diatom Skeletonema marinoi. Mol Ecol 19:4478–4490
Tahvanainen P, Alpermann TJ, Figueroa RI, John U, Hakanen P et al (2012) patterns of post-glacial genetic differentiation in marginal populations of a marine microalga. Plos One 1:e53602. https://doi.org/10.1371/journal.pone.0053602
Ellegaard M, Godhe A, Ribeiro S (2018) Time capsules in natural sediment archives-Tracking phytoplankton population genetic diversity and adaptation over multidecadal timescales in the face of environmental change. Evol Appl 11:11–16
McQuoid M, Hobson LA (1995) Importance of resting stages in diatom seasonal succession. Eur J Phycol 31:44–50
Sundqvist L, Godhe A, Jonsson RP, Sefbom J (2018) The anchoring effect—long-term dormancy and genetic population structure. ISME J 12:2929–2941
Ellegaard M, Ribeiro S (2018) The long-term persistence of phytoplankton resting stages in aquatic ‘seed banks.’ Biol Rev 93:166–183. https://doi.org/10.1111/brv.12338
Trottet A, Wilson B, Xin GSW, George C, Casten L, Schmoker C, Rawi NSBM, Siew MC, Larsen O, Eikaas SH, Tun K, Drillet G (2018) Resting stage of plankton diversity from singapore coastal water: implications for harmful algae blooms and coastal management. Environ Manag 61:275–290
Anil AC, Mitbavkar S, DeSilva MS, Hegde S, DeCosta PM, Meher SS, Banerjee D (2007) Effect of aging on survival of benthic diatom propagules. J Exp Mar Biol Ecol 343(1):37–43
Härnström K, Ellegaard M, Andersen TJ, Godhe A (2011) Hundred years of genetic structure in a sediment revived diatom population. Proc Nat Acad Sci 108:4252–4257
Ribeiro S, Berje T, Lundholm N, Andersen TJ, Abrantes F, Ellegaard M (2011) Phytoplankton growth after a century of dormancy illuminates past resilience to catastrophic darkness. Nat Commun 2:311
McQuoid M (2002) Pelagic and benthic environmental controls on the spatial distribution of a viable diatom prapagule bank of the Swedish west. J Phycol 38:881–893
Mcquoid M, Godhe A, Nordberg K (2002) Viability of phytoplankton resting stages in the sediments of a coastal Swedish fjord. Eur J Phycol 37:190–201
McQuoid M, Godhe A (2004) Recruitment of coastal planktonic diatoms from benthic versus pelagic cells: variation in bloom development and species composition. Limnol Oceanogr 49(4):1123–1133
Lewis J, Harris ASD, Jones KJ, Edmonds RL (1999) Long-term survival of marine planktonic diatoms and dinoflagellates in stored sediment samples. J Plank Res 21:343–354
Riaux-Gobin C (1996) Ditylum brightwellii (Bacillariophyceae): resting spores at the surface of a shallow sediment (Gulf of Lions, Mediterranean) and revival tests. Phycologia 35:368–371
Imai I, Itakura S, Itoh K (1990) Distribution of diatom resting cells in sediments of Harima-Nada and Northern Hiroshima Bay, the Seto Inland Sea, Japan. Bull Coast Oceanogr 28:75–84
Patil JS, Anil AC (2008) Temporal variation of diatom benthic propagules in a monsoon-influenced tropical estuary. Cont Shelf Res 28:2404–2414
Casabianca S, Capellacci S, Ricci F, Andreoni F, Russo T, Scardi M, Penna A (2020) Structure and environmental drivers of phytoplanktonic resting stage assemblages in the central Mediterranean Sea. Mar Ecol Prog Ser 639:73–89
Fukai Y, Matsuno K, Fujiwara A, Yamaguchi A (2019) The community composition of diatom resting stages in sediments of the northern Bering Sea in 2017 and 2018: the relationship to the interannual changes in the extent of the sea ice. Polar Biol 42:1915–1922
Tsukazaki C, Ishii K, Matsuno K, Yamaguchi A, Imai I (2018) Distribution of viable resting stage cells of diatoms in sediments and water columns of the Chukchi Sea, Arctic Ocean. Phycologia 57:440–452
Sicko-Goad L, Stoermer EF, Fahnenstiel G (1986) Rejuvenation of Melosira granulata (Bacillariophyceae) resting cells from the anoxic sediments of Douglas Lake, Michigan. I. Light microscopy and 14C uptake. J Phycol 22:22–28
Eilertsen HC, Sandberg S, Tøllefsen H (1995) Photoperiodic control of diatom spore growth: a theory to explain the onset of phytoplankton blooms. Mar Ecol Prog Ser 116:303–307
McQuoid M (2005) Influence of salinity on seasonal germination of resting stages and composition of microplankton on the Swedish west coast. Mar Ecol Prog Ser 289:151–163
McQuoid M, Hobson LA (1996) Diatom resting stages. J Phycol 32:889–902
Ishikawa A, Furuya K (2004) The role of diatom resting stages in the onset of the spring bloom in the East China Sea. Mar Biol 145:633–639
Itakura S, Imai I, Itoh K (1997) “Seed bank" of coastal planktonic diatoms in bottom sediments of iroshima Bay, Seto Inland Sea, Japan. Mar Biol 128:497–508
Kremp A (2001) Effects of cyst resuspension on germination and seeding of two bloom-forming dinoflagellates in the Baltic Sea. Mar Ecol Prog Ser 216:57–66
Montresor M, Prisco CD, Sarno D, Margiotta F, Zingone A (2013) Diversity and germination patterns of diatom resting stages at a coastal Mediterranean site. Mar Ecol Prog Ser 484:79–95
Rodrigues RV, Patil JS (2021) Salinity changes may influence dinoflagellate cyst morphometry: data from monsoon-influenced tropical coastal ecosystems. J. Plankton Res 43(6):853–864
Härnström K, Godhe A, Saravanan V, Karunasagar I, Karunasagar I, Sofi Rehnstam-Holm A (2007) Tropical phytoplankton community development in mesocosms inoculated with different life stages. Mar Ecol Prog Ser 346:75–88
Patil JS, Anil AC (2011) Variations in phytoplankton community in a monsoon-influenced tropical estuary. Environ Monit Assess 182:191–200
Patil JS, Anil AC (2015) Effect of monsoonal perturbations on the occurrence of phytoplankton blooms in a tropical bay. Mar Ecol Prog Ser 530:77–92
Vijith V, Sundar D, Shetye SR (2009) Time-dependence of salinity in monsoonal estuaries. Estuar Coast Shelf Sci 85:601–608
Piredda R, Sarno D, Lange BC, Tomasino PM, Zingone A, Montresor M (2017) Diatom resting stages in surface sediments: a pilot study comparing next generation sequencing and serial dilution cultures. Crypt Algol 38(1):31–46
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can J Microbiol 8:229–239
Patil JS, Anil AC (2019) Assessment of phytoplankton photo-physiological status from a tropical monsoonal estuary. Ecol Indicat 103:289–300
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for sea water analysis. Pergmon Press, Oxford
Patil JS, Anil AC (2019) Simulation experiments to elucidate variable fluorescence as a potential proxy for bulk microalgal viability from natural water, sediments and biofilms: Implication in ships ballast water management. J Environ Manag 222:242–249
Tomas C (1997) Identifying marine phytoplankton. Academic Press, San Diego, CA
Burge DRL, Edlund BM, Frisch D (2018) Paleolimnology and resurrection ecology: the future of reconstructing the past. Evol Appl 11:42–59
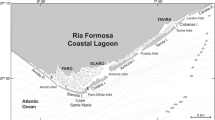
Shetye S, Murty C (1987) Seasonal variation of the salinity in the Zuari estuary, Goa, India. J Earth Syst Sci 96:249–257
Sundar D, Unnikrishnan AS, Michael GS, Kankonkar A, Nidheesh AG, Subeesh MP (2015) Observed variations in stratification and currents in the Zuari estuary, west coast of India. Environ Earth Sci 74:6951–6965
Rajaneesh KM, Mitbavkar S (2013) Factors controlling the temporal and spatial variations in Synechococcus abundance in a monsoonal estuary. Mar Environ Res 92:133–143
Pelusi A, Santelia EM, Benvenuto G, Godhe A, Montresor M (2020) The diatom Chaetoceros socialis: spore formation and preservation. Eur J Phycol 55(1):1–10
Pelusi A, Margiotta F, Passarelli A, Ferrante IM, d’Alcalà RM, Montresor M (2020) Density-dependent mechanisms regulate spore formation in the diatom Chaetoceros socialis. Limnol Oceanogr Lett. https://doi.org/10.1002/lol2.10159
Kuwata A, Takahashi M (1999) Survival of resting spores and resting cells of the marine planktonic diatom Chaetoceros pseudocurvicetus under fluctuating nitrate conditions. Mar Biol 134:471–478
Shikata T, Nukata A, Yoshikawa S, Matsubara T et al (2009) Effects of light quality on initiation and development of meroplanktonic diatom blooms in a eutrophic shallow sea. Mar Biol 156:875–889
Shikata T, Iseki M, Matsunaga S, Higashi Y, Kamei Y, Watanabe M (2011) Blue and red light-induced germination of resting spores in the red-tide diatom Leptocylindrus danicus. Photochemist Photobiol 87:590–597
Pednekar SM, Matondkar SGP, Gomes HDR, Goes JI, Parab S, Kerkar V (2011) Fine-scale responses of phytoplankton to freshwater influx in a tropical monsoonal estuary following the onset of southwest monsoon. J Earth Syst Sci 120(3):545–556
Pednekar SM, Kerkar V, Matondkar SGP (2014) Spatiotemporal distribution in phytoplankton community with distinct salinity regimes along the Mandovi estuary, Goa, India. Turk J Bot 38:808–818
Mochemadkar S, Gauns M, Pratihary A, Thorat B, Roy R, Pai IK, Naqvi SWA (2013) Response of phytoplankton to nutrient enrichment with high growth rates in a tropical monsoonal estuary - Zuari estuary, India. Ind J Geo-Mar Sci 42(3):314–325
Hollibaugh JT, Seibert DLR, Thomas WH (1981) Observation on the survival and germination of resting spores of three Chaetoceros (Bacillariophycea) species. J Phycol 17:1–9
Shikata T, Nagasoe S, Matsubara T, Yoshikawa S et al (2008) Factors influencing the initiation of blooms of the raphidophyte Heterosigma akashiwo and the diatom Skeletonema costatum in a port in Japan. Limnol Oceanogr 53:2503–2518
Jewson DH, Granin NG, Zhdanov AA, Gorbunova LA, Bondarenko NA, Gnatovsky RY (2008) Resting stages and ecology of the planktonic diatom Aulacoseira skvortzowii in Lake Baikal. Limnol Oceanogr 53:1125–1136
French FW, Hargraves PE (1985) Spore formation in the life cycles of the diatoms Chaetoceros diadema and Leptocylindrus danicus. J Phycol 21:477–483
Anderson OR (1975) The ultrastructure and cytochemistry of resting cell formation in Amphora coffeaeformis (Bacillariophyceae). J Phycol 1:272–281
DeSousa SN (1999) Effect of mining rejects on the nutrient chemistry of Mandovi Estuary, Goa. Indian J Mar Sci 28:355–359
SubhaAnand S, Sardessai S, Muthukumar C, Mangalaa KR, Sundar D, Parab SG, DileepKumar M (2014) Intra- and inter-seasonal variability of nutrients in a tropical monsoonal estuary (Zuari, India). Cont Shelf Res 82:9–30
Acknowledgements
The authors are grateful to the Director, CSIR-National Institute of Oceanography (Goa, India), and Dr. AC Anil for their support and encouragement. JSP acknowledges the Department of Science and Technology, Government of India, for the SERC young scientist project award. We acknowledge two anonymous reviewers for their constructive suggestions to improve the manuscript. This paper is NIO contribution number 6913.
Funding
SERC, DST, Government of India
Author information
Authors and Affiliations
Contributions
Sathish K performed all the experiments and statistical analysis, data interpretation; JS Patil, original concept, experiment planning, data interpretation, manuscript elaboration, and also supervised all works.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable as this study is not related to humans and animals
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, J.S., Sathish, K. Responses of Phytoplankton Benthic Propagules to Macronutrient Enrichment and Varying Light Intensities: Elucidation from Monsoon-Influenced Mandovi and Zuari Riverine System. Microb Ecol 85, 1367–1381 (2023). https://doi.org/10.1007/s00248-022-02021-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-022-02021-9