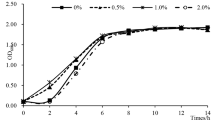
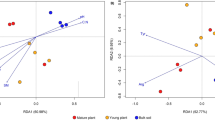
Abstract
Legume-cereal intercropping systems, in the context of diversity, ecological function, and better yield have been widely studied. Such systems enhance nutrient phytoavailability by balancing root-rhizosphere interactions. Root exudates (RE) play an important role in the rhizospheric interactions of plant-plant and/or plant-microbiome interaction. However, the influence of the primary metabolites of RE on plant-rhizobia interactions in a legume-cereal intercrop system is not known. To understand the plant communication with rhizobia, Cajanus cajan-Zea mays intercropped plants and the broad host range legume nodulating Ensifer fredii NGR234 as the model plants and rhizobium used respectively. A metabolomics-based approach revealed a clear separation between intercropped and monocropped RE of the two plants. Intercropped C. cajan showed an increase in the myo-inositol, and proline, while intercropped Z. mays showed enhanced galactose, D-glucopyranoside, and arginine in the RE. Physiological assays of NGR234 with the RE of intercropped C. cajan exhibited a significant enhancement in biofilm formation, while intercropped Z. mays RE accelerated the bacterial growth in the late log phase. Further, using label-free proteomics, we identified a total of 2570 proteins of NGR234 covering 50% annotated protein sequences upon exposure to Z. mays RE. Furthermore, intercropped Z. mays RE upregulated bacterioferritin comigratory protein (BCP), putative nitroreductase, IlvD, LeuC, D (branched-chain amino acid proteins), and chaperonin proteins GroEL2. Identification offered new insights into the metabolome of the legume-cereal intercrop and proteome of NGR234-Z. mays interactions that underline the new molecular candidates likely to be involved in the fitness of rhizobium in the intercropping system.






Similar content being viewed by others
Data availability
The raw files for proteomics are available on Massive reference- MassIVE MSV000086912.
References
Struik PC, Kuyper TW (2017) Sustainable intensification in agriculture: the richer shade of green. Agron Sustain Dev 37(5):1–15. https://doi.org/10.1007/s13593-017-0445-7
Orrell P, Bennett AE (2013) How can we exploit above–belowground interactions to assist in addressing the challenges of food security? Front Plant Sci 4:1–11. https://doi.org/10.3389/fpls.2013.00432
Brooker RW, Bennett AE, Cong WF, Daniell TJ, George TS, Hallett PD, Hawes C, Iannetta PP, Jones HG, Karley AJ, Li L (2015) Improving intercropping: a synthesis of research in agronomy, plant physiology, and ecology. New Phytol 206(1):107–117. https://doi.org/10.1111/nph.13132
Lithourgidis AS, Dordas CA, Damalas CA, Vlachostergios D (2011) Annual intercrops: an alternative pathway for sustainable agriculture. Aust J Crop Sci 5(4):396–410
Adu-Gyamfi JJ, Myaka FA, Sakala WD, Odgaard R, Vesterager JM, Høgh-Jensen H (2007) Biological nitrogen fixation and nitrogen and phosphorus budgets in farmer-managed intercrops of maize–pigeon pea in semi-arid southern and eastern Africa. Plant soil 295(1):127–136. https://doi.org/10.1007/s11104-007-9270-0
Xue Y, Xia H, Christie P, Zhang Z, Li L, Tang C (2016) Crop acquisition of phosphorus, iron, and zinc from soil in cereal/legume intercropping systems: a critical review. Ann Bot 117(3):363–377. https://doi.org/10.1093/aob/mcv182
Glaze-Corcoran, S, Hashemi, M, Sadeghpour, A, Jahanzad, E, Afshar, RK, Liu, X, Herbert, SJ (2020) Understanding intercropping to improve agricultural resiliency and environmental sustainability. In Adv Agron (Vol. 162, pp. 199–256). Academic Press. https://doi.org/10.1016/bs.agron.2020.02.004
Garland G, Bünemann EK, Oberson A, Frossard E, Six J (2017) Plant-mediated rhizospheric interactions in maize-pigeon pea intercropping enhance soil aggregation and organic phosphorus storage. Plant Soil 415(1):37–55. https://doi.org/10.1007/s11104-016-3145-1
Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol 72:313–327. https://doi.org/10.1111/j.1574-6941.2010.00860.x
Mommer L, Kirkegaard J, van Ruijven J (2016) Root-root interactions: towards a rhizosphere framework. Trends Plant Sci 21:209–217. https://doi.org/10.1016/j.tplants.2016.01.009
Kuzyakov Y, Schneckenberger K (2004) Review of estimation of plant rhizodeposition and their contribution to soil organic matter formation. Arch Agron Soil Sci 50(1):115–132. https://doi.org/10.1080/03650340310001627658
Preece C, Pen J (2020) Opinion A return to the wild : root exudates and food security. Trends Plant Sci 25:14–21. https://doi.org/10.1016/j.tplants.2019.09.010
Liu Y, Yin X, Xiao J, Tang L, Zheng Y (2019) Interactive influences of intercropping by nitrogen on flavonoid exudation and nodulation in faba bean. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-41146-9
Li B, Li YY, Wu HM, Zhang FF, Li CJ, Li XX, Lambers H, Li L (2016) Root exudates drive interspecific facilitation by enhancing nodulation and N2 fixation. Proc Natl Acad Sci 113:6496–6501. https://doi.org/10.1073/pnas.1523580113
Tian B, Pei Y, Huang W, Ding J, Siemann E (2021) Increasing flavonoid concentrations in root exudates enhance associations between arbuscular mycorrhizal fungi and an invasive plant. ISME J 15(7):1919–1930. https://doi.org/10.1038/s41396-021-00894-1
Jacoby RP, Koprivova A, Kopriva S (2021) Pinpointing secondary metabolites that shape the composition and function of the plant microbiome. J Exp Bot 72(1):57–69. https://doi.org/10.1093/jxb/eraa424
Canarini A, Kaiser C, Merchant A, Richter A, Wanek W (2019) Root exudation of primary metabolites: mechanisms and their roles in plant responses to environmental stimuli. Front Plant Sci 10:157. https://doi.org/10.3389/fpls.2019.00157
Poole P, Ramachandran V, Terpolilli J (2018) Rhizobia: from saprophytes to endosymbionts. Nat Rev Microbiol 16(5):291. https://doi.org/10.1038/nrmicro.2017.171
Masson-Boivin C, Sachs JL (2018) Symbiotic nitrogen fixation by rhizobia—the roots of a success story. Curr Opin Plant Biol 44:7–15. https://doi.org/10.1016/j.pbi.2017.12.001
Dunn MF (2015) Key roles of microsymbiont amino acid metabolism in rhizobia-legume interactions. Crit Rev Microbiol 41(4):411–451. https://doi.org/10.3109/1040841X.2013.856854
Oburger E, Jones DL (2018) Sampling root exudates–mission impossible? Rhizosphere 6:116–133. https://doi.org/10.1016/j.rhisph.2018.06.004
Rosenblueth M, Martínez-Romero E (2004) Rhizobium etli maize populations and their competitiveness for root colonization. Arch Microbiol 181(5):337–344. https://doi.org/10.1007/s00203-004-0661-9
Wu Q, Peng X, Yang M, Zhang W, Dazzo FB, Uphoff N, Jing Y, Shen S (2018) Rhizobia promote the growth of rice shoots by targeting cell signaling, division, and expansion. Plant Mol Biol 97:507–523. https://doi.org/10.1007/s11103-018-0756-3
Cavalcanti MIP, de Carvalho Nascimento R, Rodrigues DR, Escobar IEC, Fraiz ACR, de Souza AP, de Freitas ADS, Nóbrega RSA, Fernandes-Júnior PI (2020) Maize growth and yield promoting endophytes isolated into a legume root nodule by a cross-over approach. Rhizosphere 15:100211. https://doi.org/10.1016/j.rhisph.2020.100211
Van Deynze A, Zamora P, Delaux PM, Heitmann C, Jayaraman D, Rajasekar S, Graham D, Maeda J, Gibson D, Schwartz KD, Berry AM (2018) Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLoS Biol 16(8):e2006352. https://doi.org/10.6084/m9.figshare.6534545
Faget M, Nagel KA, Walter A, Herrera JM, Jahnke S, Schurr U, Temperton VM (2013) Root–root interactions: extending our perspective to be more inclusive of the range of theories in ecology and agriculture using in-vivo analyses. Ann Bot 112(2):253–266. https://doi.org/10.1093/aob/mcs296
Chen J, Arafat Y, Wu L, Xiao Z, Li Q, Khan MA, Khan MU, Lin S, Lin W (2018) Shifts in soil microbial community, soil enzymes and crop yield under peanut/maize intercropping with reduced nitrogen levels. Appl Soil Ecol 124:327–334. https://doi.org/10.1016/j.apsoil.2017.11.010
Solanki MK, Wang Z, Wang FY, Li CN, Gupta CL, Singh RK, Malviya MK, Singh P, Yang LT, Li YR (2020) Assessment of diazotrophic proteobacteria in sugarcane rhizosphere when intercropped with legumes (peanut and soybean) in the field. Front Microbiol 11:1814. https://doi.org/10.3389/fmicb.2020.01814
Gómez-Godínez LJ, Fernandez-Valverde SL, Romero JCM, Martínez-Romero E (2019) Metatranscriptomics and nitrogen fixation from the rhizoplane of maize plantlets inoculated with a group of PGPRs. Appl Microbiol 42(4):517–525. https://doi.org/10.1016/j.syapm.2019.05.003
Hara S, Morikawa T, Wasai S, Kasahara Y, Koshiba T, Yamazaki K, Fujiwara T, Tokunaga T, Minamisawa K (2019) Identification of nitrogen-fixing Bradyrhizobium associated with roots of field-grown sorghum by metagenome and proteome analyses. Front Microbiol 10:407. https://doi.org/10.3389/fmicb.2019.00407
Pueppke SG, Broughton WJ (1999) Rhizobium sp. Strain NGR234 and R fredii USDA257 share exceptionally broad, nested host ranges. Mol Plant Microbe Interact 12(4):293–318. https://doi.org/10.1094/MPMI.1999.12.4.293
Patel JK, Archana G (2017) Diverse culturable diazotrophic endophytic bacteria from Poaceae plants show cross-colonization and plant growth promotion in wheat. Plant Soil 417:99–116. https://doi.org/10.1007/s11104-017-3244-7
Hoagland, DR., & Arnon, DI (1950) The water-culture method for growing plants without soil. Circular. California agricultural experiment station, 347(2nd edit).
Huang J, Li Y, Shi Y, Wang L, Zhou Q, Huang X (2019) Effects of nutrient level and planting density on population relationship in soybean and wheat intercropping populations. PLoS ONE 14(12):e0225810. https://doi.org/10.1371/journal.pone.0225810
Vora SM, Joshi P, Belwalkar M, Archana G (2021) Root exudates influence chemotaxis and colonization of diverse plant growth-promoting rhizobacteria in the pigeon pea- maize intercropping system. Rhizosphere 18:100331. https://doi.org/10.1016/j.rhisph.2021.100331
Carvalhais LC, Dennis PG, Fedoseyenko D, Hajirezaei MR, Borriss R, von Wirén N (2011) Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J Plant Nutr Soil Sci 174(1):3–11. https://doi.org/10.1002/jpln.201000085
Ankati S, Podile AR (2019) Metabolites in the root exudates of groundnut change during interaction with plant growth promoting rhizobacteria in a strain-specific manner. J Plant Physiol 243:153057. https://doi.org/10.1016/j.jplph.2019.153057
Xia J, Wishart DS (2011) Web-based inference of biological patterns, functions, and pathways from metabolomic data using MetaboAnalyst. Nat Protoc 6:743–760. https://doi.org/10.1038/nprot.2011.319
Heberle H, Meirelles GV, da Silva FR, Telles GP, Minghim R (2015) InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics 16(1):1–7. https://doi.org/10.1186/s12859-015-0611-3
Koo, B J, Adriano, D C, Bolan, N S, & Barton, CD (2005) Root exudates and microorganisms. In ‘Encyclopedia of Soils in the Environment’(Ed D Hillel) pp. 421–428. https://doi.org/10.1016/B0-12-348530-4/00461-6
Broughton WJ, Wong CH, Lewin A, Samrey U, Myint H, Meyer H, Dowling DN, Simon R (1986) Identification of Rhizobium plasmid sequences involved in recognition of Psophocarpus, Vigna, and other legumes. J Cell Biol 102:1173–1182. https://doi.org/10.1083/jcb.102.4.1173
Robertsen BK, Åman P, Darvill AG, McNeil M, Albersheim P (1981) Host-symbiont interactions: V. The structure of acidic extracellular polysaccharides secreted by Rhizobium leguminosarum and Rhizobium trifolii. Plant Physiol 67(3):389–400. https://doi.org/10.1104/pp.67.3.389
Lee HI, Lee JH, Park KH, Sangurdekar D, Chang WS (2012) Effect of soybean coumestrol on Bradyrhizobium japonicum nodulation ability, biofilm formation, and transcriptional profile. Appl Environ Microbiol 78:2896–2903. https://doi.org/10.1128/AEM.07336-11
Wang D, Marschner P, Solaiman Z, Rengel Z (2007) Growth, P uptake and rhizosphere properties of intercropped wheat and chickpea in soil amended with iron phosphate or phytate. Soil Biol Biochem 39:249–256. https://doi.org/10.1016/j.soilbio.2006.07.013
Meng L, Zhang A, Wang F, Han X, Wang D, Li S (2015) Arbuscular mycorrhizal fungi and rhizobium facilitate nitrogen uptake and transfer in soybean/maize intercropping system. Front Plant Sci 6:1–10. https://doi.org/10.3389/fpls.2015.00339
Gomes DF, Batista JSDS, Schiavon AL et al (2012) Proteomic profiling of Rhizobium tropici PRF 81: Identification of conserved and specific responses to heat stress. BMC Microbiol 30(12):84. https://doi.org/10.1186/1471-2180-12-84
Faurobert M, Pelpoir E, Chaïb J (2007) Phenol extraction of proteins for proteomic studies of recalcitrant plant tissues. Plant Proteomics Humana Press 335:9–14. https://doi.org/10.1385/1-59745-227-0:9
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Pérez-Jaramillo JE, Mendes R, Raaijmakers JM (2016) Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol Biol 90:635–644. https://doi.org/10.1007/s11103-015-0337-7
Kerdraon L, Balesdent MH, Barret M, Laval V, Suffert F (2019) Crop residues in wheat-oilseed rape rotation system: a pivotal, shifting platform for microbial meetings. Microb Ecol 77(4):931–945. https://doi.org/10.1007/s00248-019-01340-8
Kobayashi H, Naciri-Graven Y, Broughton WJ, Perret X (2004) Flavonoids induce temporal shifts in gene-expression of nod-box controlled loci in Rhizobium sp. NGR234. Mol Microbiol 51:335–347. https://doi.org/10.1046/j.1365-2958.2003.03841.x
Staehelin C, Forsberg LS, D’Haeze W, Gao MY, Carlson RW, Xie ZP, Pellock BJ, Jones KM, Walker GC, Streit WR, Broughton WJ (2006) Exo-oligosaccharides of Rhizobium sp. strain NGR234 are required for symbiosis with various legumes. J Bacteriol 188(17):6168–6178. https://doi.org/10.1128/JB.00365-06
Khaosaad T, Staehelin C, Steinkellner S, Hage-Ahmed K, Ocampo JA, Garcia-Garrido JM, Vierheilig H (2010) The Rhizobium sp. strain NGR234 systemically suppresses arbuscular mycorrhizal root colonization in a split-root system of barley (Hordeum vulgare). Physiol Plant 140:238–245. https://doi.org/10.1111/j.1399-3054.2010.01396.x
Liu Y, Yin X, Xiao J, Tang L, Zheng Y (2019) Interactive influences of intercropping by nitrogen on flavonoid exudation and nodulation in faba bean. Sci Rep 9(1):1–11. https://doi.org/10.1038/s41598-019-41146-91
Ramakrishna W, Yadav R, Li K (2019) Plant growth-promoting bacteria in agriculture : two sides of a coin. Appl Soil Ecol 138:10–18. https://doi.org/10.1016/j.apsoil.2019.02.019
Jain, A, Singh, HB, & Das, S (2020) Deciphering plant-microbe crosstalk through proteomics studies. Microbiol Res 126590https://doi.org/10.1016/j.micres.2020.126590
Jayakumar, A, Nair, IC, & Radhakrishnan, EK (2020) Environmental adaptations of an extremely plant beneficial Bacillus subtilis Dcl1 identified through the genomic and metabolomic analysis. Microb Ecol, 1-16https://doi.org/10.1007/s00248-020-01605-7
Bacic A, Moody SF, Clarke AE (1986) Structural analysis of secreted root slime from maize ( Zea mays L.). Plant Physiol 80:771–777. https://doi.org/10.1104/pp.80.3.771
Rinaudi L, Fujishige NA, Hirsch AM, Banchio E (2006) Effects of nutritional and environmental conditions on Sinorhizobium meliloti biofilm formation 157:867–875. https://doi.org/10.1016/j.resmic.2006.06.002
He X, Chang W, Pierce DL, Seib LO, Wagner J, Fuqua C (2003) Quorum sensing in Rhizobium sp. strain NGR234 regulates conjugal transfer (tra) gene expression and influences growth rate. J Bacteriol 185(3):809–822. https://doi.org/10.1128/JB.185.3.809
Calatrava-Morales N, McIntosh M, Soto MJ (2018) Regulation mediated by N-acyl homoserine lactone quorum-sensing signals in the rhizobium-legume symbiosis. Genes 9(5):263. https://doi.org/10.3390/genes9050263
Skøt L, Egsgaard H (1984) Identification of ononitol and O-methyl-scyllo-inositol in pea root nodules. Planta 161:32–36. https://doi.org/10.1007/BF00951457
Naveed M, Brown LK, Raffan AC, George TS, Bengough AG, Roose T, Sinclair I, Koebernick N, Cooper L, Hackett CA, Hallett PD (2017) Plant exudates may stabilize or weaken soil depending on species, origin and time. Eur J Soil Sci 68(6):806–816. https://doi.org/10.1111/ejss.12487
Poole P, Blyth A, Reid CJ, Walters K (2019) Myo-inositol catabolism and catabolite regulation in Rhizobium leguminosarum bv. viciae. Microbiol 140:2787–2795. https://doi.org/10.1099/00221287-140-10-2787
Fry J, Wood M, Poole PS (2001) Investigation of myo -Inositol Catabolism in Rhizobium leguminosarum bv. viciae and its effect on nodulation competitiveness. Mol Plant Microbe Interact 14:1016–1025. https://doi.org/10.1094/MPMI.2001.14.8.1016
Ding H, Yip CB, Geddes BA, Oresnik IJ, Hynes MF, Tn AB (2019) Glycerol utilization by Rhizobium leguminosarum requires an ABC transporter and affects competition for nodulation. Microbiol 158:1369–1378. https://doi.org/10.1099/mic.0.057281-0
Lodwig EM, Hosie AH, Bourdes A, Findlay K, Allaway D, Karunakaran R, Downie JA, Poole PS (2003) Amino-acid cycling drives nitrogen fixation in the legume–Rhizobium symbiosis. Nature 422(6933):722–726. https://doi.org/10.1038/nature01527
Lv, J, Dong, Y, Dong, K, Zhao, Q, Yang, Z, Chen, L (2020) Intercropping with wheat suppressed Fusarium wilt in faba bean and modulated the composition of root exudates. Plant Soil 1-12https://doi.org/10.1007/s11104-019-04413-2
Gosai, J, Anandhan, S, Bhattacharjee, A, and Archana, G (2019) Elucidation of quorum sensing components and their role in the regulation of symbiotically important traits in Ensifer nodulating pigeon pea. Microbiol Res. 231, 126354. https:// doi:https://doi.org/10.1016/j.micres.2019.126354
Kuznetsov VV, Rakitin VY, Zholkevich VN (1999) Effects of preliminary heat-shock treatment on accumulation of osmolytes and drought resistance in cotton plants during water deficiency. Physiol Plant 107:399–406. https://doi.org/10.1034/j.1399-3054.1999.100405.x
Dhont C, Castonguay Y, Nadeau P, Bélanger G, Drapeau R, Laberge S, Avice JC, Chalifour FP (2006) Nitrogen reserves, spring regrowth and winter survival of field-grown alfalfa (Medicago sativa) defoliated in the autumn. Ann Bot 97:109–120. https://doi.org/10.1093/aob/mcj006
Liang Y, Cao Y, Tanaka K, Thibivilliers S, Wan J, Choi J, ho Kang, C, Qiu, J, Stacey, G, (2013) Nonlegumes respond to rhizobial Nod factors by suppressing the innate immune response. Science 341(6152):1384–1387. https://doi.org/10.1126/science.1242736
Le Strange, KK, Bender, GL, Djordjevic, MA, Rolfe, BG, & Redmond, J W (1990) The Rhizobium strain NGR234 nodD1 gene product responds to activation by the simple phenolic compounds vanillin and isovanillin present in wheat seedling extracts. Mol Plant-Microbe Interact 3:214. https://doi:https://doi.org/10.1094/MPMI-3-214
Ogawa J, Long SR (1995) The Rhizobium meliloti groELc locus is required for regulation of early nod genes by the transcription activator. NodD. Genes Dev 9(6):714–729. https://doi.org/10.1101/gad.9.6.714
Reddy PM, Ladha JK, So RB, Hernandez RJ, Ramos MC, Angeles OR, Dazzo FB, de Bruijn FJ (1997) Rhizobial communication with rice roots: induction of phenotypic changes, mode of invasion and extent of colonization. Plant Soil 194:81–98. https://doi.org/10.1007/978-94-011-7113-7_9
Rinaudi LV, Giordano W (2010) An integrated view of biofilm formation in rhizobia. FEMS Microbiol Lett 304:1–11. https://doi.org/10.1111/j.1574-6968.2009.01840.x
Pérez-Montaño F, Jiménez-Guerrero I, Del Cerro P, Baena-Ropero I, López-Baena FJ, Ollero FJ, Bellogín R, Lloret J, Espuny R (2014) The symbiotic biofilm of Sinorhizobium fredii SMH12, necessary for successful colonization and symbiosis of Glycine max cv Osumi, is regulated by quorum sensing systems and inducing flavonoids via NodD1. PLoS ONE 9(8):e105901. https://doi.org/10.1371/journal.pone.0105901
Reyes-Pérez A, del Carmen Vargas M, Hernández M, Aguirre-von-Wobeser E, Pérez-Rueda E, Encarnacion S (2016) Transcriptomic analysis of the process of biofilm formation in Rhizobium etli CFN42. Arch Microbiol 198(9):847–860. https://doi.org/10.1007/s00203-016-1241-5
Chang C, Damiani I, Puppo A, Frendo P (2009) Redox changes during the legume–Rhizobium symbiosis. Mol Plant 2(3):370–377. https://doi.org/10.1093/mp/ssn090
Benyamina SM, Baldacci-Cresp F, Couturier J, Chibani K, Hopkins J, Bekki A, de Lajudie P, Rouhier N, Jacquot JP, Alloing G, Puppo A (2013) Two Sinorhizobium meliloti glutaredoxins regulate iron metabolism and symbiotic bacteroid differentiation. Environ Microbiol 15(3):795–810. https://doi.org/10.1111/j.1462-2920.2012.02835.x
Nanda AK, Andrio E, Marino D, Pauly N, Dunand C (2010) Reactive oxygen species during plant-microorganism early interactions. J Integr Plant Biol 52(2):195–204. https://doi.org/10.1111/j.1744-7909.2010.00933.x
Liu X, Qiu W, Rao B, Cao Y, Fang X, Yang J, Jiang G, Zhong Z, Zhu J (2019) Bacterioferritin comigratory protein is important in hydrogen peroxide resistance, nodulation, and nitrogen fixation in Azorhizobium caulinodans. Arch Microbiol 201(6):823–831. https://doi.org/10.1007/s00203-019-01654-8
de Oliveira IM, Zanotto-Filho A, Moreira JCF, Bonatto D, Henriques JAP (2010) The role of two putative nitroreductases, Frm2p and Hbn1p, in the oxidative stress response in Saccharomyces cerevisiae. Yeast 27(2):89–102. https://doi.org/10.1002/yea
Chen WM, Prell J, James EK, Sheu DS, Sheu SY (2012) Biosynthesis of branched-chain amino acids is essential for effective symbioses between betarhizobia and Mimosa pudica. Microbiology 158(7):1758–1766. https://doi.org/10.1099/mic.0.058370-0
Okazaki S, Hattori Y, Saeki K (2007) The Mesorhizobium loti purB gene is involved in infection thread formation and nodule development in Lotus japonicus. J Bacteriol 189:8347–8352. https://doi.org/10.1128/JB.00788-07
Li T, Zhan Z, Lin Y, Lin M, Xie Q, Chen Y, He C, Tao J, Li C (2019) Biosynthesis of amino acids in Xanthomonas oryzae pv. oryzae is essential to its pathogenicity. Microorganisms 7(12):693. https://doi.org/10.3390/microorganisms7120693
Ferraioli S, Tatè R, Cermola M, Favre R, Iaccarino M, Patriarca EJ (2002) Auxotrophic mutant strains of Rhizobium etli reveal new nodule development phenotypes. Mol Plant Microbe Interact 15:501–510. https://doi.org/10.1094/MPMI.2002.15.5.501
Ramachandran, VK, East, AK, Karunakaran, R, Downie, JA, Poole, PS (2011) Adaptation of Rhizobium leguminosarum to pea, alfalfa, and sugar beet rhizosphere investigated by comparative transcriptomics. Genome Biol 12, R106. https://doi:https://doi.org/10.1186/gb-2011-12-10-r106
Krysciak D, Schmeisser C, Preuss S, Riethausen J, Quitschau M, Grond S, Streit WR (2011) Involvement of multiple loci in quorum quenching of autoinducer I molecules in the nitrogen-fixing symbiont Rhizobium (Sinorhizobium) sp. strain NGR234. Appl Environ Microbiol 77(15):5089–5099. https://doi.org/10.1128/AEM.00112-11
Li Y, Tian CF, Chen WF, Wang L, Sui XH, Chen WX (2013) High-resolution transcriptomic analyses of Sinorhizobium sp. NGR234 bacteroids in determinate nodules of Vigna unguiculata and indeterminate nodules of Leucaena leucocephala. PloS one 8(8):e70531. https://doi.org/10.1371/journal.pone.0070531
Acknowledgements
The authors are grateful to Pulse Research Station (Vadodara) and Dr. S. K. Singh from Main Maize Research Station (Godhra) of Anand Agricultural University, India for providing C. cajan and Z. mays seeds. We acknowledge Dr. Murali Sharaff, PDPIAS, CHARUSAT, Changa for providing needful suggestions to the manuscript. We gratefully acknowledge the Metabolomics facility, the School of Life Sciences, the University of Hyderabad for GC-MS/MS analysis, and the Centre for Cellular and Molecular Platforms (C-CAMP), Bangalore for LC-MS/MS facility.
Funding
SMV is grateful to the University Grant Commission, New Delhi, India for the UGC-BSR fellowship. ARP thanks the Science and Engineering Research Board, Department of Science and Technology, Govt. of India for the JC Bose Fellowship (Grant No. JCB/2017/000053). The infrastructure support to the Department of Microbiology and Biotechnology Centre at The M.S. University of Baroda, Vadodara under the DST-FIST program of Govt. of India is acknowledged.
Author information
Authors and Affiliations
Contributions
SMV performed the research and interpreted the data; GA and SMV designed the experiments, analyzed the data, and wrote the manuscript. SA and ARP standardized the method and analyzed metabolomics (GC–MS/MS) data. SA and ARP also provided critical comments on the write-up of the manuscript. CP performed label-free quantitative proteomics (LC/MS/MS) and also carried out data analysis. All authors gave final approval of the version to be submitted and any revised version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable, since this article does not involve any studies with human participants or animals by any of the authors.
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vora, S.M., Ankati, S., Patole, C. et al. Alterations of Primary Metabolites in Root Exudates of Intercropped Cajanus cajan–Zea mays Modulate the Adaptation and Proteome of Ensifer (Sinorhizobium) fredii NGR234. Microb Ecol 83, 1008–1025 (2022). https://doi.org/10.1007/s00248-021-01818-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-021-01818-4