Abstract
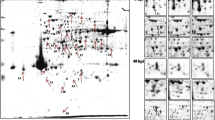
Rigidoporus microporus is the fungus accountable for the white root rot disease that is detrimental to the rubber tree, Hevea brasiliensis. The pathogenicity mechanism of R. microporus and the identity of the fungal proteins and metabolites involved during the infection process remain unclear. In this study, the protein and metabolite profiles of two R. microporus isolates, Segamat (SEG) and Ayer Molek (AM), were investigated during an in vitro interaction with H. brasiliensis. The isolates were used to inoculate H. brasiliensis clone RRIM 2025, and mycelia adhering to the roots of the plant were collected for analysis. Transmission electron microscope (TEM) images acquired confirms the hyphae attachment and colonization of the mycelia on the root of the H. brasiliensis clones after 4 days of inoculation. The protein samples were subjected to 2-DE analysis and analyzed using MALDI-ToF MS/MS, while the metabolites were extracted using methanol and analyzed using LC/MS-QTOF. Based on the differential analyses, upregulation of proteins that are essential for fungal evolution such as malate dehydrogenase, fructose 1,6-biphosphate aldolase, and glyceraldehyde-3-phosphate dehydrogenase hints an indirect role in fungal pathogenicity, while metabolomic analysis suggests an increase in acidic compounds which may lead to increased cell wall degrading enzyme activity. Bioinformatics analyses revealed that the carbohydrate and amino acid metabolisms were prominently affected in response to the fungal pathogenicity. In addition to that, other pathways that were significantly affected include “Protein Ubiquitination Pathway,” Unfolded Protein Response,” “HIFα Signaling,” and “Sirtuin Signaling Pathway.” The identification of responsive proteins and metabolites from this study promotes a better understanding of mechanisms underlying R. microporus pathogenesis and provides a list of potential biological markers for early recognition of the white root rot disease.









Similar content being viewed by others
Data Availability
The nucleotide sequences of all test genes were submitted in the NCBI Genbank database under accession numbers. Rigidoporus microporus Ayer Molek (AM) (accession No. MG199552.1) and Segamat (SEG) (accession No. MG199553.1); Hevea brasiliensis (accession No. MF981856.1).
References
Monkai J, Hyde KD, Xu J, Mortimer PE (2017) Diversity and ecology of soil fungal communities in rubber plantations. Fungal Biol Rev 31:1–11
Malaysian Rubber Board Digest (2020) Nat. RUBBER Stat. Malaysian Rubber Board 1–29. http://www3.lgm.gov.my/digest/digest/digest-1-2020.pdf
Haryuni, Suwandi (2003) Mode of dispersal and variation in population of white root fungus Rigidoporus microporus as revealed by mycelial incompatibility. In: Biosantifikasi. Salatiga, Indonesia, pp 68–75
Shabbir I, Abd Samad MY, Othman R et al (2020) White root rot disease suppression in rubber plant with microbial co-inoculants and silicon addition. Rhizosphere 15:1–8. https://doi.org/10.1016/j.rhisph.2020.100221
Chaiharn M, Sujada N, Pathom-Aree W, Lumyong S (2019) Biological control of Rigidoporus microporus the cause of white root disease in rubber using PGPRs in vivo. Chiang Mai J Sci 46:850–866
Farhana AHKF, Bahri ARS, Thanh TAV, Zakaria L (2017) Morphological features of Rigidoporus microporus isolated from infected Malaysian rubber clones. Malaysian J Microsc 13:17–23
Islam MR, Tudryn G, Bucinell R et al (2017) Morphology and mechanics of fungal mycelium. Sci Rep 7:13070. https://doi.org/10.1038/s41598-017-13295-2
Riquelme M, Aguirre J, Bartnicki-García S et al (2018) Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. MicrobiolMolBiol Rev 82:1–47. https://doi.org/10.1128/mmbr.00068-17
Pandey V, Singh M, Pandey D, Kumar A (2018) Integrated proteomics, genomics, metabolomics approaches reveal oxalic acid as pathogenicity factor in Tilletia indica inciting Karnal bunt disease of wheat. Sci Rep 8:1–14. https://doi.org/10.1038/s41598-018-26257-z
Tan BC, Lim YS, Lau SE (2017) Proteomics in commercial crops: an overview. J Proteomics 169:176–188. https://doi.org/10.1016/j.jprot.2017.05.018
Al-Obaidi JR, Saidi NB, Usuldin SRA, et al (2016) Differential proteomic study of oil palm leaves in response to in vitro inoculation with pathogenic and non-pathogenic Ganoderma spp. J Plant Pathol 98:33–42. https://doi.org/10.4454/JPP.V98I2.025
Al-Obaidi JR, HussinSaidi SNISNB et al (2017) Comparative proteomic analysis of Ganoderma species during in vitro interaction with oil palm root. PhysiolMol Plant Pathol 99:16–24. https://doi.org/10.1016/j.pmpp.2017.02.001
Al-Obaidi JR, Saidi NB, Usuldin SRA et al (2016) Comparison of different protein extraction methods for gel-based proteomic analysis of Ganodermaspp. Protein J 35:100–106. https://doi.org/10.1007/s10930-016-9656-z
Martínez-salgado JL, León-ramírez CG, Barrera A et al (2013) Analysis of the regulation of the Ustilagomaydis proteome by dimorphism, pH or MAPK and GCN5 genes. J Proteomics 79:251–262
Manikandan R, Karthikeyan G, Raguchander T (2017) Soil proteomics for exploitation of microbial diversity in Fusarium wilt infected and healthy rhizosphere soils of tomato. PhysiolMol Plant Pathol 100:185–193. https://doi.org/10.1016/j.pmpp.2017.10.001
Wanichthanarak K, Fahrmann JF, Grapov D (2015) Genomic, proteomic, and metabolomic data integration strategies. Biomark Insights 10:1–6. https://doi.org/10.4137/BMI.S29511.TYPE
Alizadeh MA, Jafari AA, Sepahvand K et al (2021) Evaluation of sainfoin accessions exposed to powdery mildew disease at four locations in Iran. Trop Grassl-Forrajes Trop 9:97–108. https://doi.org/10.17138/TGFT(9)97-108
Srivastava S (2019) Emerging insights into the metabolic alterations in aging using metabolomics. Metabolites 9:1–16. https://doi.org/10.3390/metabo9120301
Hong J, Yang L, Zhang D, Shi J (2016) Plant metabolomics: an indispensable system biology tool for plant science. Int J Mol Sci 17:767. https://doi.org/10.3390/ijms17060767
Tizro P, Choi C, Khanlou N (2019) Sample preparation for transmission electron microscopy. Methods MolBiol 1897:417–424. https://doi.org/10.1007/978-1-4939-8935-5_33
Jamil NAM, Rahmad N, Rosli NHM, Al-Obaidi JR (2018) Proteomic and metabolomic study of wax apple (Syzygium samarangense) fruit during ripening process. Electrophoresis. https://doi.org/10.1002/elps.201800185
Razali N, Aziz AA, Lim CY, Junit SM (2015) Investigation into the effects of antioxidant-rich extract of Tamarindus indica leaf on antioxidant enzyme activities, oxidative stress and gene expression profiles in HepG2 cells. PeerJ. https://doi.org/10.7717/peerj.1292
Baysal Ö, Lai D, Xu HH et al (2013) A proteomic approach provides new insights into the control of soil-borne plant pathogens by bacillus species. PLoS ONE. https://doi.org/10.1371/journal.pone.0053182
Zhang P, Zhu Y, Luo X, Zhou S (2019) Comparative proteomic analysis provides insights into the complex responses to Pseudoperonosporacubensis infection of cucumber (Cucumis sativus L.). Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-45111-4
Kia SH, Jurkechova M, Glynou K et al (2018) The effects of fungal root endophytes on plant growth are stable along gradients of abiotic habitat conditions. FEMS MicrobiolEcol 94:1–10. https://doi.org/10.1093/femsec/fix162
Pawlowski ML, Hartman GL (2016) Infection mechanisms and colonization patterns of fungi associated with soybean. Fungal Pathog. https://doi.org/10.5772/62305
Peyraud R, Dubiella U, Barbacci A et al (2017) Advances on plant–pathogen interactions from molecular toward systems biology perspectives. Plant J 90:720–737. https://doi.org/10.1111/tpj.13429
Rodriguez-Moreno L, Ebert MK, Bolton MD, Thomma BPHJ (2018) Tools of the crook- infection strategies of fungal plant pathogens. Plant J 93:664–674. https://doi.org/10.1111/tpj.13810
Quecine MC, Leite TF, Bini AP et al (2016) Label-free quantitative proteomic analysis of Puccinia psidiiuredospores reveals differences of fungal populations infecting eucalyptus and guava. PLoS ONE 11:1–19. https://doi.org/10.1371/journal.pone.0145343
González-Fernández R, Aloria K, Valero-Galván J et al (2014) Proteomic analysis of mycelium and secretome of different Botrytis cinerea wild-type strains. J Proteomics 97:195–221. https://doi.org/10.1016/j.jprot.2013.06.022
Ibáñez AB, Bauer S (2014) Analytical method for the determination of organic acids in dilute acid pretreated biomass hydrolysate by liquid chromatography-time-of-flight mass spectrometry. Biotechnol Biofuels 7:1–9. https://doi.org/10.1186/s13068-014-0145-3
Tong K, Li Zl, Sun X et al (2017) Metabolomics approach reveals annual metabolic variation in roots of Cyathula officinalisKuan based on gas chromatography-mass spectrum. Chinese Med (United Kingdom) 12:1–10. https://doi.org/10.1186/s13020-017-0133-1
Ziveri J, Tros F, Guerrera IC et al (2017) The metabolic enzyme fructose-1,6 bisphosphate aldolase acts as a transcriptional regulator in pathogenic Francisella. Nat Commun 8:1–15. https://doi.org/10.1038/s41467-017-00889-7
Luong TTM, Wang WW, Zhang F et al (2019) Structure-antifungal relationships and preventive effects of 1-(2,4-dihydroxyphenyl)-2-methylpropan-1-one derivatives as potential inhibitors of class-II fructose-1,6-bisphosphate aldolase. PesticBiochemPhysiol 159:41–50. https://doi.org/10.1016/j.pestbp.2019.05.016
Pachauri S, Chatterjee S, Kumar V, Mukherjee PK (2019) A dedicated glyceraldehyde-3-phosphate dehydrogenase is involved in the biosynthesis of volatile sesquiterpenes in Trichoderma virens—evidence for the role of a fungal GAPDH in secondary metabolism. Curr Genet 65:243–252. https://doi.org/10.1007/s00294-018-0868-y
Mecejana A, Souza D, Regina L et al (2017) Estimates of genetic parameters for the rubber yield and secondary traits in rubber tree. Ind Crop Prod 98:19–24. https://doi.org/10.1016/j.indcrop.2017.01.017
Lima JO, Pereira JF, Rincones J et al (2009) The glyceraldehyde-3-phosphate dehydrogenase gene of Moniliophthoraperniciosa, the causal agent of witches’ broom disease of Theobroma cacao. Genet MolBiol 32:362–366. https://doi.org/10.1590/S1415-47572009000200024
Gao T, Chen J, Shi Z (2016) Fusarium graminearum Pyruvate dehydrogenase kinase 1 (FgPDK1) is critical for conidiation, mycelium growth, and pathogenicity. PLoS ONE 11:1–19. https://doi.org/10.1371/journal.pone.0158077
Tiwari S, Thakur R, Shankar J (2015) Role of heat-shock proteins in cellular function and in the biology of fungi. Biotechnol Res Int 2015:1–11. https://doi.org/10.1155/2015/132635
Chen L, Geng X, Ma Y et al (2019) The ER lumenal Hsp70 protein FpLhs1 is important for conidiation and plant infection in Fusarium pseudograminearum. Front Microbiol 10:1–12. https://doi.org/10.3389/fmicb.2019.01401
Tauzin, A.S., Giardina, T.:Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front. Plant Sci. (2014)
Vylkova S (2017) Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoSPathog 13:1–6. https://doi.org/10.1371/journal.ppat.1006149
Pusztahelyi, T., Holb, I.J., Pócsi, I.:Plant-fungal interactions: special secondary metabolites of the biotrophic, necrotrophic, and other specific interactions. In: Jean-Michel MérillonKishan GR (ed) Fungal metabolites. pp 1–58 (2016)
Meir Z, Osherov N (2018) Vitamin biosynthesis as an antifungal target. J Fungi 4:72. https://doi.org/10.3390/jof4020072
Zhang DD, Wang XY, Chen JY et al (2016) Identification and characterization of a pathogenicity-related gene VdCYP1 from Verticillium dahliae. Sci Rep 6:1–12. https://doi.org/10.1038/srep27979
Acknowledgements
The authors would like to thank the Malaysian Rubber Board
Funding
This research was funded by the Malaysian Rubber Board grant number (S16IPDM0533).
Author information
Authors and Affiliations
Contributions
Jameel R. Al-Obaidi conceptualized and designed the research. Jameel R. Al-Obaidi, Noor Baity Saidi, Roslinda Sajari, Dhilia Udie Lamasudin, and Safiah Atan executed the research. Ahmad Faiz Bin Che Fisol run the experiments and wrote the paper. Jameel R. Al-Obaidi, Ahmad Faiz Bin Che Fisol, and Norasfaliza Rahmad performed the proteomic analysis. Siti Nahdatul Isnaini Said Hussin and Nurul Hafiza MR performed the plant challenge experiment. Roslinda Sajari performed microscopy. Nurhanani Razali performed the bioinformatics analysis. All authors agree on the final content of this manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The work presented has not been published before in any language or submitted to another journal for consideration.
Consent to Participate
The authors have given informed consent regarding the submission of the manuscript.
Conflict of Interest
The authors declare no competing interests.
Research Involving Human and Animal Rights
No humans or animals were used in this research.
Rights and permissions
About this article
Cite this article
Fisol, A.F.B.C., Saidi, N.B., Al-Obaidi, J.R. et al. Differential Analysis of Mycelial Proteins and Metabolites From Rigidoporus Microporus During In Vitro Interaction With Hevea Brasiliensis. Microb Ecol 83, 363–379 (2022). https://doi.org/10.1007/s00248-021-01757-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-021-01757-0