Abstract
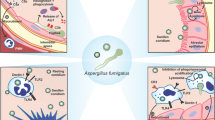
Invasive aspergillosis and scedosporiosis are life-threatening fungal infections with similar clinical manifestations in immunocompromised patients. Contrarily, Scedosporium apiospermum is susceptible to some azole derivative but often resistant to amphotericin B. Histopathological examination alone cannot diagnose these two fungal species. Pathogenesis studies could contribute to explore candidate protein markers for new diagnosis and treatment methods leading to a decrease in mortality. In the present study, proteomics was conducted to identify significantly altered proteins in A549 cells infected with or without Aspergillus fumigatus and S. apiospermum as measured at initial invasion. Protein validation was performed with immunogold labelling alongside immunohistochemical techniques in infected A549 cells and lungs from murine models. Further, cytokine production was measured, using the Bio-Plex-Multiplex immunoassay. The cytoskeletal proteins HSPA9, PA2G4, VAT1, PSMA2, PEX1, PTGES3, KRT1, KRT9, CLIP1 and CLEC20A were mainly changed during A. fumigatus infection, while the immunologically activated proteins WNT7A, GAPDH and ANXA2 were principally altered during S. apiospermum infection. These proteins are involved in fungal internalisation and structural destruction leading to pulmonary disorders. Interleukin (IL)-21, IL-1α, IL-22, IL-2, IL-8, IL-12, IL-17A, interferon-γ and tumour necrosis factor-α were upregulated in both aspergillosis and scedosporiosis, although more predominately in the latter, in accordance with chitin synthase-1 and matrix metalloproteinase levels. Our results demonstrated that during invasion, A. fumigatus primarily altered host cellular integrity, whereas S. apiospermum chiefly induced and extensively modulated host immune responses.









Similar content being viewed by others
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Yu WL, Liu WL, Chan KS, Yang CC, Tan CK, Tsai CL, Chen CM, Chuang YC (2018) High-level ambient particulate matter before influenza attack with increased incidence of Aspergillus antigenemia in Southern Taiwan, 2016. J Microbiol Immunol Infect 51:141–147. https://doi.org/10.1016/j.jmii.2016.09.001
Guarner J, Brandt ME (2011) Histopathologic diagnosis of fungal infections in the 21st century. Clin. Microbiol. Rev. 24:247–280. https://doi.org/10.1128/CMR.00053-10
Revie NM, Iyer KR, Robbins N, Cowen LE (2018) Antifungal drug resistance: evolution, mechanisms and impact. Curr. Opin. Microbiol. 45:70–76. https://doi.org/10.1016/j.mib.2018.02.005
Braunsdorf C, LeibundGut-Landmann S (2018) Modulation of the fungal-host interaction by the intra-species diversity of C. albicans. Pathogens 7. https://doi.org/10.3390/pathogens7010011
Guarro J, Kantarcioglu AS, Horre R, Rodriguez-Tudela JL, Cuenca Estrella M, Berenguer J, de Hoog GS (2006) Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med. Mycol. 44:295–327. https://doi.org/10.1080/13693780600752507
Liu H, Lee MJ, Solis NV, Phan QT, Swidergall M, Ralph B, Ibrahim AS, Sheppard DC, Filler SG (2016) Aspergillus fumigatus CalA binds to integrin alpha5beta1 and mediates host cell invasion. Nat. Microbiol. 2:16211. https://doi.org/10.1038/nmicrobiol.2016.211
Orciuolo E, Stanzani M, Canestraro M, Galimberti S, Carulli G, Lewis R, Petrini M, Komanduri KV (2007) Effects of Aspergillus fumigatus gliotoxin and methylprednisolone on human neutrophils: implications for the pathogenesis of invasive aspergillosis. J. Leukoc. Biol. 82:839–848. https://doi.org/10.1189/jlb.0207090
van de Veerdonk FL, Gresnigt MS, Romani L, Netea MG, Latge JP (2017) Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 15:661–674. https://doi.org/10.1038/nrmicro.2017.90
Amitani R, Taylor G, Elezis EN, Llewellyn-Jones C, Mitchell J, Kuze F, Cole PJ, Wilson R (1995) Purification and characterization of factors produced by Aspergillus fumigatus which affect human ciliated respiratory epithelium. Infect. Immun. 63:3266–3271
Arias M, Santiago L, Vidal-Garcia M, Redrado S, Lanuza P, Comas L, Domingo MP, Rezusta A, Galvez EM (2018) Preparations for invasion: modulation of host lung immunity during pulmonary aspergillosis by gliotoxin and other fungal secondary metabolites. Front. Immunol. 9:2549. https://doi.org/10.3389/fimmu.2018.02549
Sales-Campos H, Tonani L, Cardoso CRB, Kress MRV (2013) The immune interplay between the host and the pathogen in Aspergillus fumigatus lung infection. Biomed. Res. Int.:Artn 693023. https://doi.org/10.1155/2013/693023
Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C (2009) Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 182:4938–4946. https://doi.org/10.4049/jimmunol.0804250
Porter PC, Roberts L, Fields A, Knight M, Qian Y, Delclos GL, Han S, Kheradmand F, Corry DB (2011) Necessary and sufficient role for T helper cells to prevent fungal dissemination in allergic lung disease. Infect. Immun. 79:4459–4471. https://doi.org/10.1128/IAI.05209-11
Liu W, Feng RZ, Jiang HL (2019) Scedosporium spp lung infection in immunocompetent patients: a systematic review and MOOSE-compliant meta-analysis. Medicine (Baltimore) 98:e17535. https://doi.org/10.1097/MD.0000000000017535
Aor AC, Mello TP, Sangenito LS, Fonseca BB, Rozental S, Lione VF, Veiga VF, Branquinha MH, Santos AL (2018) Ultrastructural viewpoints on the interaction events of Scedosporium apiospermum conidia with lung and macrophage cells. Mem. Inst. Oswaldo Cruz 113:e180311. https://doi.org/10.1590/0074-02760180311
Silva BA, Pinto MR, Soares RM, Barreto-Bergter E, Santos AL (2006) Pseudallescheria boydii releases metallopeptidases capable of cleaving several proteinaceous compounds. Res. Microbiol. 157:425–432. https://doi.org/10.1016/j.resmic.2005.11.010
Roilides E, Simitsopoulou M, Katragkou A, Walsh TJ (2009) Host immune response against Scedosporium species. Med. Mycol. 47:433–440. https://doi.org/10.1080/13693780902738006
Mina S, Staerck C, d'Almeida SM, Marot A, Delneste Y, Calenda A, Tabiasco J, Bouchara JP, Fleury MJJ (2015) Identification of Scedosporium boydii catalase A1 gene, a reactive oxygen species detoxification factor highly expressed in response to oxidative stress and phagocytic cells. Fungal Biol 119:1322–1333. https://doi.org/10.1016/j.funbio.2015.09.007
Staerck C, Vandeputte P, Gastebois A, Calenda A, Giraud S, Papon N, Bouchara JP, Fleury MJJ (2018) Enzymatic mechanisms involved in evasion of fungi to the oxidative stress: focus on Scedosporium apiospermum. Mycopathologia 183:227–239. https://doi.org/10.1007/s11046-017-0160-6
Al Refai M, Duhamel C, Le Rochais JP, Icard P (2002) Lung scedosporiosis: a differential diagnosis of aspergillosis. Eur. J. Cardiothorac. Surg. 21:938–939. https://doi.org/10.1016/s1010-7940(02)00068-4
Salehi E, Hedayati MT, Zoll J, Rafati H, Ghasemi M, Doroudinia A, Abastabar M, Tolooe A, Snelders E, van der Lee HA, Rijs AJ, Verweij PE, Seyedmousavi S, Melchers WJ (2016) Discrimination of aspergillosis, mucormycosis, fusariosis, and scedosporiosis in formalin-fixed paraffin-embedded tissue specimens by use of multiple real-time quantitative PCR assays. J. Clin. Microbiol. 54:2798–2803. https://doi.org/10.1128/JCM.01185-16
Cagas SE, Jain MR, Li H, Perlin DS (2011) The proteomic signature of Aspergillus fumigatus during early development. Mol. Cell. Proteomics 10:M111 010108. https://doi.org/10.1074/mcp.M111.010108
Cagas SE, Jain MR, Li H, Perlin DS (2011) Profiling the Aspergillus fumigatus proteome in response to caspofungin. Antimicrob. Agents Chemother. 55:146–154. https://doi.org/10.1128/AAC.00884-10
Curty N, Kubitschek-Barreira PH, Neves GW, Gomes D, Pizzatti L, Abdelhay E, Souza GH, Lopes-Bezerra LM (2014) Discovering the infectome of human endothelial cells challenged with Aspergillus fumigatus applying a mass spectrometry label-free approach. J. Proteome 97:126–140. https://doi.org/10.1016/j.jprot.2013.07.003
da Silva BA, Sodre CL, Souza-Goncalves AL, Aor AC, Kneipp LF, Fonseca BB, Rozental S, Romanos MT, Sola-Penna M, Perales J, Kalume DE, dos Santos AL (2012) Proteomic analysis of the secretions of Pseudallescheria boydii, a human fungal pathogen with unknown genome. J. Proteome Res. 11:172–188. https://doi.org/10.1021/pr200875x
Kniemeyer O, Lessing F, Brakhage AA (2009) Proteome analysis for pathogenicity and new diagnostic markers for Aspergillus fumigatus. Med. Mycol. 47(Suppl 1):S248–S254. https://doi.org/10.1080/13693780802169138
Voltersen V, Blango MG, Herrmann S, Schmidt F, Heinekamp T, Strassburger M, Kruger T, Bacher P, Lother J, Weiss E, Hunniger K, Liu H, Hortschansky P, Scheffold A, Loffler J, Krappmann S, Nietzsche S, Kurzai O, Einsele H, Kniemeyer O, Filler SG, Reichard U, Brakhage AA (2018) Proteome analysis reveals the conidial surface protein CcpA essential for virulence of the pathogenic fungus Aspergillus fumigatus. mBio 9:9. https://doi.org/10.1128/mBio.01557-18
Luplertlop N, Pumeesat P, Muangkaew W, Wongsuk T, Alastruey-Izquierdo A (2016) Environmental screening for the Scedosporium apiospermum species complex in public parks in Bangkok, Thailand. PLoS One 11:e0159869. https://doi.org/10.1371/journal.pone.0159869
Gonzalez GM, Tijerina R, Najvar LK, Bocanegra R, Rinaldi MG, Loebenberg D, Graybill JR (2003) Activity of posaconazole against Pseudallescheria boydii: in vitro and in vivo assays. Antimicrob. Agents Chemother. 47:1436–1438
Lelievre B, Legras P, Godon C, Franconi F, Saint-Andre JP, Bouchara JP, Diquet B (2013) Experimental models of disseminated scedosporiosis with cerebral involvement. J. Pharmacol. Exp. Ther. 345:198–205. https://doi.org/10.1124/jpet.112.201541
Gonzalez GM, Tijerina R, Najvar L, Rinaldi M, Yeh IT, Graybill JR (2002) Experimental murine model of disseminated Pseudallescheria infection. Med. Mycol. 40:243–248
Rodriguez MM, Pastor FJ, Salas V, Calvo E, Mayayo E, Guarro J (2010) Experimental murine scedosporiosis: histopathology and azole treatment. Antimicrob. Agents Chemother. 54:3980–3984. https://doi.org/10.1128/AAC.00046-10
Ampawong S, Luplertlop N (2019) Experimental scedosporiosis induces cerebral oedema associated with abscess regarding aquaporin-4 and Nrf-2 depletions. Biomed. Res. Int.:Artn 6076571. https://doi.org/10.1155/2019/6076571
Ampawong S, Isarangkul D, Reamtong O, Aramwit P (2018) Adaptive effect of sericin on hepatic mitochondrial conformation through its regulation of apoptosis, autophagy and energy maintenance: a proteomics approach. Sci. Rep. 8:ARTN 14943. https://doi.org/10.1038/s41598-018-33372-4
Zhang J, Jiang H, Du Y, Keyhani NO, Xia Y, Jin K (2019) Members of chitin synthase family in Metarhizium acridum differentially affect fungal growth, stress tolerances, cell wall integrity and virulence. PLoS Pathog. 15:e1007964. https://doi.org/10.1371/journal.ppat.1007964
Ampawong S, Aramwit P (2016) Tolerogenic responses of CD206+, CD83+, FOXP3+, and CTLA-4 to sericin/polyvinyl alcohol/glycerin scaffolds relevant to IL-33 and HSP60 activity. Histol. Histopathol. 31:1011–1027. https://doi.org/10.14670/HH-11-733
Ampawong S, Combes V, Hunt NH, Radford J, Chan-Ling T, Pongponratn E, Grau GE (2011) Quantitation of brain edema and localisation of aquaporin 4 expression in relation to susceptibility to experimental cerebral malaria. Int. J. Clin. Exp. Pathol. 4:566–574
Ampawong S, Isarangkul D, Aramwit P (2017) Sericin improves heart and liver mitochondrial architecture in hypercholesterolaemic rats and maintains pancreatic and adrenal cell biosynthesis. Exp. Cell Res. 358:301–314. https://doi.org/10.1016/j.yexcr.2017.07.001
Ampawong S, Isarangkul D, Aramwit P (2017) Sericin ameliorated dysmorphic mitochondria in high-cholesterol diet/streptozotocin rat by antioxidative property. Exp. Biol. Med. 242:411–421. https://doi.org/10.1177/1535370216681553
Tiwari S, Thakur R, Shankar J (2015) Role of heat-shock proteins in cellular function and in the biology of fungi. Biotechnol. Res. Int. 2015:132635–132611. https://doi.org/10.1155/2015/132635
Li WQ, Hu XC, Zhang XH, Ge YP, Zhao SN, Hu Y, Ashman RB (2011) Immunisation with the glycolytic enzyme enolase confers effective protection against Candida albicans infection in mice. Vaccine 29:5526–5533. https://doi.org/10.1016/j.vaccine.2011.05.030
Takaoka Y, Goto S, Nakano T, Tseng HP, Yang SM, Kawamoto S, Ono K, Chen CL (2014) Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) prevents lipopolysaccharide (LPS)-induced, sepsis-related severe acute lung injury in mice. Sci. Rep. 4:5204. https://doi.org/10.1038/srep05204
Ko HR, Nguyen TLX, Kim CK, Park Y, Lee KH, Ahn JY (2015) P42 Ebp1 functions as a tumor suppressor in non-small cell lung cancer. BMB Rep. 48:159–165. https://doi.org/10.5483/BMBRep.2015.48.3.130
Alka K, Casey JR (2014) Bicarbonate transport in health and disease. IUBMB Life 66:596–615. https://doi.org/10.1002/iub.1315
Bae S, Lee EM, Cha HJ, Kim K, Yoon Y, Lee H, Kim J, Kim YJ, Lee HG, Jeung HK, Min YH, An S (2011) Resveratrol alters microRNA expression profiles in A549 human non-small cell lung cancer cells. Mol. Cell 32:243–249. https://doi.org/10.1007/s10059-011-1037-z
Kan F, Ye L, Yan T, Cao J, Zheng J, Li W (2017) Proteomic and transcriptomic studies of HBV-associated liver fibrosis of an AAV-HBV-infected mouse model. BMC Genomics 18:641. https://doi.org/10.1186/s12864-017-3984-z
Chamberlain N, Korwin-Mihavics BR, Nakada EM, Bruno SR, Heppner DE, Chapman DG, Hoffman SM, van der Vliet A, Suratt BT, Dienz O, Alcorn JF, Anathy V (2019) Lung epithelial protein disulfide isomerase A3 (PDIA3) plays an important role in influenza infection, inflammation, and airway mechanics. Redox Biol. 22:101129. https://doi.org/10.1016/j.redox.2019.101129
Zafar S, Asif AR, Ramljak S, Tahir W, Schmitz M, Zerr I (2014) Anchorless 23-230 PrPC interactomics for elucidation of PrPC protective role. Mol. Neurobiol. 49:1385–1399. https://doi.org/10.1007/s12035-013-8616-2
Dias TR, Agarwal A, Pushparaj PN, Ahmad G, Sharma R (2019) Reduced semen quality in patients with testicular cancer seminoma is associated with alterations in the expression of sperm proteins. Asian J Androl 22:88–93. https://doi.org/10.4103/aja.aja_17_19
Wei CC, Guo DF, Zhang SL, Ingelfinger JR, Chan JS (2005) Heterogenous nuclear ribonucleoprotein F modulates angiotensinogen gene expression in rat kidney proximal tubular cells. J. Am. Soc. Nephrol. 16:616–628. https://doi.org/10.1681/ASN.2004080715
Stiburek L, Fornuskova D, Wenchich L, Pejznochova M, Hansikova H, Zeman J (2007) Knockdown of human Oxa1l impairs the biogenesis of F1Fo-ATP synthase and NADH:ubiquinone oxidoreductase. J. Mol. Biol. 374:506–516. https://doi.org/10.1016/j.jmb.2007.09.044
Wang C, Yan R, Luo D, Watabe K, Liao DF, Cao D (2009) Aldo-keto reductase family 1 member B10 promotes cell survival by regulating lipid synthesis and eliminating carbonyls. J. Biol. Chem. 284:26742–26748. https://doi.org/10.1074/jbc.M109.022897
Koch J, Foekens J, Timmermans M, Fink W, Wirzbach A, Kramer MD, Schaefer BM (2003) Human VAT-1: a calcium-regulated activation marker of human epithelial cells. Arch. Dermatol. Res. 295:203–210. https://doi.org/10.1007/s00403-003-0421-8
Luong TTM, Wang WW, Zhang F, Dan WJ, Nien HX, Zhang AL, Li D, Gao JM (2019) Structure-antifungal relationships and preventive effects of 1-(2,4-dihydroxyphenyl)-2-methylpropan-1-one derivatives as potential inhibitors of class-II fructose-1,6-bisphosphate aldolase. Pestic. Biochem. Physiol. 159:41–50. https://doi.org/10.1016/j.pestbp.2019.05.016
Patipong T, Ngoennet S, Honda M, Hibino T, Waditee-Sirisattha R, Kageyama H (2019) A class I fructose-1,6-bisphosphate aldolase is associated with salt stress tolerance in a halotolerant cyanobacterium Halothece sp. PCC 7418. Arch. Biochem. Biophys. 672:108059. https://doi.org/10.1016/j.abb.2019.07.024
Li Y, Huang J, Sun J, Xiang S, Yang D, Ying X, Lu M, Li H, Ren G (2017) The transcription levels and prognostic values of seven proteasome alpha subunits in human cancers. Oncotarget 8:4501–4519. https://doi.org/10.18632/oncotarget.13885
Colasante C, Chen JP, Ahlemeyer B, Bonilla-Martinez R, Karnati S, Baumgart-Vogt E (2017) New insights into the distribution, protein abundance and subcellular localisation of the endogenous peroxisomal biogenesis proteins PEX3 and PEX19 in different organs and cell types of the adult mouse. PLoS One 12:ARTN e0183150. https://doi.org/10.1371/journal.pone.0183150
Chang TS, Lin HK, Rogers KA, Brame LS, Yeh MM, Yang Q, Fung KM (2013) Expression of aldo-keto reductase family 1 member C3 (AKR1C3) in neuroendocrine tumors & adenocarcinomas of pancreas, gastrointestinal tract, and lung. Int. J. Clin. Exp. Pathol. 6:2419–2429
Hawkins GA, Smith RS, Moore WC, Peters SP, Meyers DA, Bleecker ER, Program NSAR (2009) Glucocorticoid receptor hetero-complex gene STIP1 is associated with measures of lung function: data from the Severe Asthma Research Program (SARP). Am J Resp Crit Care 179.
Zhang C, Chen F, Liu X, Han X, Hu Y, Su X, Chen Y, Sun Y, Han L (2019) Gliotoxin induces cofilin phosphorylation to promote actin cytoskeleton dynamics and internalization of Aspergillus fumigatus into type II human pneumocyte cells. Front. Microbiol. 10:1345. https://doi.org/10.3389/fmicb.2019.01345
Kononikhin AS, Brzhozovskiy AG, Ryabokon AM, Fedorchenko K, Zhakharova NV, Spasskii AI, Popov IA, Ilyin VK, Solovyova ZO, Pastushkova LK, Polyakov AV, Varfolomeev SD, Larina IM, Nikolaev EN (2019) Proteome profiling of the exhaled breath condensate after long-term spaceflights. Int. J. Mol. Sci. 20:20. https://doi.org/10.3390/ijms20184518
Preising M, Ayuso C (2004) Rab escort protein 1 (REP1) in intracellular traffic: a functional and pathophysiological overview. Ophthalmic Genet. 25:101–110. https://doi.org/10.1080/13816810490514333
Xue R, Wan Y, Zhang S, Zhang Q, Ye H, Li Y (2014) Role of bone morphogenetic protein 4 in the differentiation of brown fat-like adipocytes. Am. J. Physiol. Endocrinol. Metab. 306:E363–E372. https://doi.org/10.1152/ajpendo.00119.2013
Longo LV, Nakayasu ES, Matsuo AL, Peres da Silva R, Sobreira TJ, Vallejo MC, Ganiko L, Almeida IC, Puccia R (2013) Identification of human plasma proteins associated with the cell wall of the pathogenic fungus Paracoccidioides brasiliensis. FEMS Microbiol. Lett. 341:87–95. https://doi.org/10.1111/1574-6968.12097
Joshi N, Johnson LL, Wei WQ, Abnet CC, Dong ZW, Taylor PR, Limburg PJ, Dawsey SM, Hawk ET, Qiao YL, Kirsch IR (2006) Gene expression differences in normal esophageal mucosa associated with regression and progression of mild and moderate squamous dysplasia in a high-risk Chinese population. Cancer Res. 66:6851–6860. https://doi.org/10.1158/0008-5472.CAN-06-0662
Alam H, Sehgal L, Kundu ST, Dalal SN, Vaidya MM (2011) Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol. Biol. Cell 22:4068–4078. https://doi.org/10.1091/mbc.E10-08-0703
Roth W, Kumar V, Beer HD, Richter M, Wohlenberg C, Reuter U, Thiering S, Staratschek-Jox A, Hofmann A, Kreusch F, Schultze JL, Vogl T, Roth J, Reichelt J, Hausser I, Magin TM (2012) Keratin 1 maintains skin integrity and participates in an inflammatory network in skin through interleukin-18. J. Cell Sci. 125:5269–5279. https://doi.org/10.1242/jcs.116574
Wang LL, Lee KT, Jung KW, Lee DG, Bahn YS (2018) The novel microtubule-associated CAP-glycine protein Cgp1 governs growth, differentiation, and virulence of Cryptococcus neoformans. Virulence 9:566–584. https://doi.org/10.1080/21505594.2017.1423189
Butin-Israeli V, Adam SA, Goldman AE, Goldman RD (2012) Nuclear lamin functions and disease. Trends Genet. 28:464–471. https://doi.org/10.1016/j.tig.2012.06.001
Kasabova M, Villeret B, Gombault A, Lecaille F, Reinheckel T, Marchand-Adam S, Couillin I, Lalmanach G (2016) Discordance in cathepsin B and cystatin C expressions in bronchoalveolar fluids between murine bleomycin-induced fibrosis and human idiopathic fibrosis. Respir. Res. 17:ARTN 118. https://doi.org/10.1186/s12931-016-0432-6
Kruzel ML, Zimecki M, Actor JK (2017) Lactoferrin in a context of inflammation-induced pathology. Front. Immunol. 8:ARTN 1438. https://doi.org/10.3389/fimmu.2017.01438
Varadarajan S, Das A, Youn SW, Kohno T, Surenkhuu B, Ushio-Fukai M, Fukai T (2015) Copper transporter ATP7A limits vascular inflammation and aortic aneurysm development. Circulation 132.
Troilo H, Bayley CP, Barrett AL, Lockhart-Cairns MP, Jowitt TA, Baldock C (2016) Mammalian tolloid proteinases: role in growth factor signalling. FEBS Lett. 590:2398–2407. https://doi.org/10.1002/1873-3468.12287
Chen Y, Zhang H, Xu A, Li N, Liu JF, Liu CJ, Lv D, Wu S, Huang LY, Yang SY, He DC, Xiao XY (2006) Elevation of serum l-lactate dehydrogenase B correlated with the clinical stage of lung cancer. Lung Cancer 54:95–102. https://doi.org/10.1016/j.lungcan.2006.06.014
Fuentes N, Roy A, Mishra V, Cabello N, Silveyra P (2018) Sex-specific microRNA expression networks in an acute mouse model of ozone-induced lung inflammation. Biol. Sex Differ. 9:ARTN 18. https://doi.org/10.1186/s13293-018-0177-7
Wu Q, Eickelberg O (2019) Ezrin in asthma: a first step to early biomarkers of airway epithelial dysfunction. Am J Resp Crit Care 199:408–410. https://doi.org/10.1164/rccm.201810-1964ED
Gupta R, van Dongen J, Fu Y, Abdellaoui A, Tyndale RF, Velagapudi V, Boomsma DI, Korhonen T, Kaprio J, Loukola A, Ollikainen M (2019) Epigenome-wide association study of serum cotinine in current smokers reveals novel genetically driven loci. Clin. Epigenetics 11:ARTN 1. https://doi.org/10.1186/s13148-018-0606-9
Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S (2014) PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 211:781–790. https://doi.org/10.1084/jem.20131916
Seth P, Csizmadia E, Hedblom A, Vuerich M, Xie H, Li M, Longhi MS, Wegiel B (2017) Deletion of lactate dehydrogenase-A in myeloid cells triggers antitumor immunity. Cancer Res. 77:3632–3643. https://doi.org/10.1158/0008-5472.CAN-16-2938
Stukes S, Coelho C, Rivera J, Jedlicka AE, Hajjar KA, Casadevall A (2016) The membrane phospholipid binding protein annexin A2 promotes phagocytosis and nonlytic exocytosis of cryptococcus neoformans and impacts survival in fungal infection. J. Immunol. 197:1252–1261. https://doi.org/10.4049/jimmunol.1501855
Winn RA, Van Scoyk M, Hammond M, Rodriguez K, Crossno JT, Heasley LE, Nemenoff RA (2006) Antitumorigenic effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 281:26943–26950. https://doi.org/10.1074/jbc.M604145200
Muzio L, Brambilla V, Calcaterra L, D'Adamo P, Martino G, Benedetti F (2016) Increased neuroplasticity and hippocampal microglia activation in a mice model of rapid antidepressant treatment. Behav. Brain Res. 311:392–402. https://doi.org/10.1016/j.bbr.2016.05.063
Tavanti A, Naglik JR, Osherov N (2012) Host-fungal interactions: pathogenicity versus immunity. Int J Microbiol 2012:562480–562482. https://doi.org/10.1155/2012/562480
Desoubeaux G, Chauvin D, Piqueras MDC, Bronson E, Bhattacharya SK, Sirpenski G, Bailly E, Cray C (2018) Translational proteomic study to address host protein changes during aspergillosis. PLoS One 13:e0200843. https://doi.org/10.1371/journal.pone.0200843
Kogan TV, Jadoun J, Mittelman L, Hirschberg K, Osherov N (2004) Involvement of secreted Aspergillus fumigatus proteases in disruption of the actin fiber cytoskeleton and loss of focal adhesion sites in infected A549 lung pneumocytes. J. Infect. Dis. 189:1965–1973. https://doi.org/10.1086/420850
Roilides E, Lamaignere CG, Farmaki E (2002) Cytokines in immunodeficient patients with invasive fungal infections: an emerging therapy. Int. J. Infect. Dis. 6:154–163. https://doi.org/10.1016/s1201-9712(02)90104-9
Thompson A, Orr SJ (2018) Emerging IL-12 family cytokines in the fight against fungal infections. Cytokine 111:398–407. https://doi.org/10.1016/j.cyto.2018.05.019
Brieland JK, Jackson C, Menzel F, Loebenberg D, Cacciapuoti A, Halpern J, Hurst S, Muchamuel T, Debets R, Kastelein R, Churakova T, Abrams J, Hare R, O'Garra A (2001) Cytokine networking in lungs of immunocompetent mice in response to inhaled Aspergillus fumigatus. Infect. Immun. 69:1554–1560. https://doi.org/10.1128/IAI.69.3.1554-1560.2001
Chotirmall SH, Al-Alawi M, Mirkovic B, Lavelle G, Logan PM, Greene CM, McElvaney NG (2013) Aspergillus-associated airway disease, inflammation, and the innate immune response. Biomed. Res. Int. 2013:723129–723114. https://doi.org/10.1155/2013/723129
Gil-Lamaignere C, Winn RM, Simitsopoulou M, Maloukou A, Walsh TJ, Roilides E (2005) Inteferon gamma and granulocyte-macrophage colony-stimulating factor augment the antifungal activity of human polymorphonuclear leukocytes against Scedosporium spp.: comparison with Aspergillus spp. Med. Mycol. 43:253–260. https://doi.org/10.1080/13693780412331271072
Solaymani-Mohammadi S, Eckmann L, Singer SM (2019) Interleukin (IL)-21 in inflammation and immunity during parasitic diseases. Front. Cell. Infect. Microbiol. 9:401. https://doi.org/10.3389/fcimb.2019.00401
Reeder KM, Mackel JJ, Godwin MS, Dunaway CW, Blackburn JP, Patel RP, Steele C (2018) Role of common gamma-chain cytokines in lung interleukin-22 regulation after acute exposure to Aspergillus fumigatus. Infect. Immun. 86. https://doi.org/10.1128/IAI.00157-18
Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282–289. https://doi.org/10.1038/nm1720
De Luca A, Zelante T, D'Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F, Puccetti P, Romani L (2010) IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 3:361–373. https://doi.org/10.1038/mi.2010.22
Caffrey AK, Lehmann MM, Zickovich JM, Espinosa V, Shepardson KM, Watschke CP, Hilmer KM, Thammahong A, Barker BM, Rivera A, Cramer RA, Obar JJ (2015) IL-1 alpha signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS Pathog. 11:UNSP e1004625. https://doi.org/10.1371/journal.ppat.1004625
Deepe Jr GS, McGuinness M (2006) Interleukin-1 and host control of pulmonary histoplasmosis. J. Infect. Dis. 194:855–864. https://doi.org/10.1086/506946
Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JW, Kullberg BJ (2006) Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J. Infect. Dis. 193:1419–1426. https://doi.org/10.1086/503363
Caffrey-Carr AK, Kowalski CH, Beattie SR, Blaseg NA, Upshaw CR, Thammahong A, Lust HE, Tang YW, Hohl TM, Cramer RA, Obar JJ (2017) Interleukin 1alpha is critical for resistance against highly virulent Aspergillus fumigatus isolates. Infect. Immun. 85. https://doi.org/10.1128/IAI.00661-17
Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M (2002) Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 72:847–855
Luna-Rodriguez CE, Trevino-Rangel RJ, Soto-Dominguez A, Becerril-Garcia MA, Gonzalez-Montalvo MA, Saldivar MA, Rodriguez-Rocha H, Gonzalez GM (2020) Development of an immunocompetent murine model of pulmonary infection due to Scedosporium apiospermum. Microb. Pathog. 142:104073. https://doi.org/10.1016/j.micpath.2020.104073
Malacco NL, Souza JA, Mendes AC, Rachid MA, Kraemer LR, Mattos MS, Lima GN, Sousa LP, Souza DG, Pinho V, Teixeira MM, Russo RC, Soriani FM (2019) Acute lung injury and repair induced by single exposure of Aspergillus fumigatus in immunocompetent mice. Future Microbiol. 14:1511–1525. https://doi.org/10.2217/fmb-2019-0214
Ghannoum MA (2000) Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 13:122–143, table of contents. https://doi.org/10.1128/cmr.13.1.122-143.2000
Gago S, Overton NLD, Ben-Ghazzi N, Novak-Frazer L, Read ND, Denning DW, Bowyer P (2018) Lung colonization by Aspergillus fumigatus is controlled by ZNF77. Nat. Commun. 9:ARTN 3835. https://doi.org/10.1038/s41467-018-06148-7
Acknowledgements
Additional support was kindly provided by Northeast Laboratory Animal Center, Khon Kaen University, and Faculty of Tropical Medicine and Faculty of Science, Mahidol University.
Funding
This study was funded by the National Research Council of Thailand and Mahidol University under the research project “Pulmonary invasive role of Scedosporium apiospermum possible receptor and signalling for diagnosis and adjunctive treatment.” Additional funds were provided by TSRI Fund (CU_FRB640001_01_33_1), the Health System Research Institute (HSRI 63-005) and the Asia Research Center of the Korean Foundation, Chulalongkorn University (011/2560).
Author information
Authors and Affiliations
Contributions
All authors participated in the design, interpretation and analysis of the studies. All authors conducted the experiments as follows: proteomics studies by DI, OR and TT; in vitro and in vivo studies by NS, PS, WM and SA; histopathological and electron microscopic analyses by TK, KF and SA; and Bio-Plex Multiplex immunoassay by PS, WM, KF and SA. All authors wrote, revised and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Animal experiments were conducted in accordance with parameters described in the Animals for Scientific Purposes Act, B.E. 2558, Thailand, and were approved by the Institutional Animal Care and Use Committee of Khon Kaen University (IACUC-KKU-20/61).
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Kanjanapruthipong, T., Sukphopetch, P., Reamtong, O. et al. Cytoskeletal Alteration Is an Early Cellular Response in Pulmonary Epithelium Infected with Aspergillus fumigatus Rather than Scedosporium apiospermum. Microb Ecol 83, 216–235 (2022). https://doi.org/10.1007/s00248-021-01750-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-021-01750-7