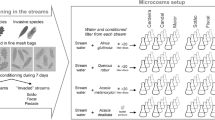
Abstract
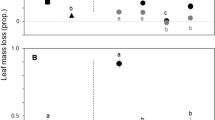
Non-native nitrogen-fixing Acacia species have been invading riparian ecosystems worldwide, potentially threatening stream communities that strongly depend on allochthonous litter. We examined the effects of the invasion of native deciduous temperate forests by Acacia species on litter decomposition and associated fungal decomposers in streams. Litter of native (Alnus glutinosa and Quercus robur) and invasive (Acacia melanoxylon) species were enclosed in fine-mesh bags and immersed in three native and three invaded streams, for 14–98 days. Litter decomposition rates, fungal biomass, and aquatic hyphomycete sporulation rates were higher in invaded than in native streams, likely due to the higher water nitrogen concentration found in invaded streams. Alnus glutinosa litter had higher aquatic hyphomycete sporulation rates and species richness, and higher decomposition rates, probably because they were soft and nitrogen rich. Quercus robur litter also had high aquatic hyphomycete sporulation rates but lower decomposition rates than Al. glutinosa, probably due to high polyphenol concentration and carbon:nitrogen ratio. Acacia melanoxylon litter had lower aquatic hyphomycete sporulation rates and species richness, and lower decomposition rates, most likely because it was very tough. Thus, litter decomposition rates varied in the order: Al. glutinosa > Q. robur > Ac. melanoxylon. The aquatic hyphomycete community structure strongly differed between native and invaded streams, and among litter species, suggesting that microbes were sensitive to water nitrogen concentration and litter characteristics. Overall, increases in water nitrogen concentration and alterations in litter characteristics promoted by the invasion of native riparian forests by Acacia species may affect the activity and community structure of microbial decomposers, and instream litter decomposition, thus altering the functioning of stream ecosystems.









Similar content being viewed by others
References
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of Earth’s ecosystems. Science 277:494–499. https://doi.org/10.1126/science.277.5325.494
Vilà M, Hulme P (2017) Impact of biological invasions on ecosystem services. Springer International Publishing, Switzerland
Castro-Díez P, Vaz AS, Silva JS, van Loo M, Alonso Á, Aponte C, Bayón Á, Bellingham PJ, Chiuffo MC, DiManno N, Julian K, Kandert S, La Porta N, Marchante H, Maule HG, Mayfield MM, Metcalfe D, Monteverdi MC, Núñez MA, Ostertag R, Parker IM, Peltzer DA, Potgieter LJ, Raymundo M, Rayome D, Reisman-Berman O, Richardson DM, Roos RE, Saldaña A, Shackleton RT, Torres A, Trudgen M, Urban J, Vicente JR, Vilà M, Ylioja T, Zenni RD, Godoy O (2019) Global effects of non-native tree species on multiple ecosystem services. Biol Rev 94:1477–1501. https://doi.org/10.1111/brv.12511
Castro-Díez P, Alonso Á (2017) Effects of non-native riparian plants in riparian and fluvial ecosystems: a review for the Iberian Peninsula. Limnetica 36:525–541. https://doi.org/10.23818/limn.36.19
Lorenzo P, González L, Reigosa MJ (2010) The genus Acacia as invader: the characteristic case of Acacia dealbata Link in Europe. Ann For Sci 67:101. https://doi.org/10.1051/forest/2009082
Souza-Alonso P, Rodríguez J, González L, Lorenzo P (2017) Here to stay. Recent advances and perspectives about Acacia invasion in Mediterranean areas. Ann For Sci 74:55. https://doi.org/10.1007/s13595-017-0651-0
Ferreira V, Castela J, Rosa P, Tonin AM, Boyero L, Graça MAS (2016) Aquatic hyphomycetes, benthic macroinvertebrates and leaf litter decomposition in streams naturally differing in riparian vegetation. Aquat Ecol 50:711–725. https://doi.org/10.1007/s10452-016-9588-x
Ferreira V, Figueiredo A, Graça MAS, Marchante E, Pereira A (2021) Invasion of temperate deciduous broadleaf forests by N-fixing tree species—consequences for stream ecosystems. Biol Rev. https://doi.org/10.1111/brv.12682
Graça MAS, Pozo J, Canhoto C, Elosegi A (2002) Effects of Eucalyptus plantations on detritus, decomposers, and detritivores in streams. ScientificWorldJournal 2:1173–1185. https://doi.org/10.1100/tsw.2002.193
Hladyz S, Åbjörnsson K, Giller PS, Woodward G (2011) Impacts of an aggressive riparian invader on community structure and ecosystem functioning in stream food webs. J Appl Ecol 48:443–452. https://doi.org/10.1111/j.1365-2664.2010.01924.x
Martínez A, Larrañaga A, Pérez J, Descals E, Basaguren A, Pozo J (2013) Effects of pine plantations on structural and functional attributes of forested streams. For Ecol Manage 310:147–155. https://doi.org/10.1016/j.foreco.2013.08.024
Ferreira V, Koricheva J, Pozo J, Graça MAS (2016) A meta-analysis on the effects of changes in the composition of native forests on litter decomposition in streams. For Ecol Manage 364:27–38. https://doi.org/10.1016/j.foreco.2016.01.002
Compton JE, Church MR, Larned ST, Hogsett WE (2003) Nitrogen export from forested watersheds in the Oregon Coast Range: the role of N2-fixing Red Alder. Ecosystems 6:773–785. https://doi.org/10.1007/s10021-002-0207-4
Goldstein CL, Williard KWJ, Schoonover JE (2009) Impact of an invasive exotic species on stream nitrogen levels in Southern Illinois. J Am Water Resour Assoc 45:664–672. https://doi.org/10.1111/j.1752-1688.2009.00314.x
Shaftel RS, King RS, Back JA (2012) Alder cover drives nitrogen availability in Kenai lowland headwater streams, Alaska. Biogeochemistry 107:135–148. https://doi.org/10.1007/s10533-010-9541-3
Wiegner TN, Hughes F, Shizuma LM, Bishaw DK, Manuel ME (2013) Impacts of an invasive N2-fixing tree on Hawaiian stream water quality. Biotropica 45:409–418. https://doi.org/10.1111/btp.12024
Singh G, Mejía NMM, Williard KWJ, Schoonover JE, Groninger JW (2019) Watershed vulnerability to invasive N2-fixing autumn olive and consequences for stream nitrogen concentrations. J Environ Qual 48:614–623. https://doi.org/10.2134/jeq2018.09.0343
Stewart SD, Young MB, Harding JS, Horton TW (2019) Invasive nitrogen-fixing plant amplifies terrestrial-aquatic nutrient flow and alters ecosystem function. Ecosystems 22:587–601. https://doi.org/10.1007/s10021-018-0289-2
Hieber M, Gessner MO (2002) Contribution of stream detrivores, fungi, and bacteria to leaf breakdown based on biomass estimates. Ecology 83:1026–1038. https://doi.org/10.1890/0012-9658(2002)083[1026:COSDFA]2.0.CO;2
Baldy V, Gobert V, Guerold F, Chauvet E, Lambrigot D, Charcosset J-Y (2007) Leaf litter breakdown budgets in streams of various trophic status: effects of dissolved inorganic nutrients on microorganisms and invertebrates. Freshw Biol 52:1322–1335. https://doi.org/10.1111/j.1365-2427.2007.01768.x
Gulis V, Su R, Kuehn KA (2019) Fungal decomposers in freshwater environments. In: Hurst CJ (ed) The structure and function of aquatic microbial communities. Advances in Environmental Microbiology. Springer International Publishing, Switzerland, pp 121–155
Ferreira V, Gulis V, Graça MAS (2006) Whole-stream nitrate addition affects litter decomposition and associated fungi but not invertebrates. Oecologia 149:718–729. https://doi.org/10.1007/s00442-006-0478-0
Ferreira V, Graça MAS (2016) Effects of whole-stream nitrogen enrichment and litter species mixing on litter decomposition and associated fungi. Limnologica 58:69–77. https://doi.org/10.1016/j.limno.2016.03.002
Rajashekhar M, Kaveriappa KM (2003) Diversity of aquatic hyphomycetes in the aquatic ecosystems of the Western Ghats of India. Hydrobiologia 501:167–177. https://doi.org/10.1023/A:1026239917232
Lecerf A, Dobson M, Dang CK, Chauvet E (2005) Riparian plant species loss alters trophic dynamics in detritus-based stream ecosystems. Oecologia 146:432–442. https://doi.org/10.1007/s00442-005-0212-3
Laitung B, Chauvet E (2005) Vegetation diversity increases species richness of leaf-decaying fungal communities in woodland streams. Arch Hydrobiol 164:217–235. https://doi.org/10.1127/0003-9136/2005/0164-0217
Canhoto C, Graça MAS (1996) Decomposition of Eucalyptus globulus leaves and three native leaf species (Alnus glutinosa, Castanea sativa and Quercus faginea) in a Portuguese low order stream. Hydrobiologia 333:79–85. https://doi.org/10.1007/BF00017570
Gulis V (2001) Are there any substrate preferences in aquatic hyphomycetes? Mycol Res 105:1088–1093. https://doi.org/10.1016/S0953-7562(08)61971-1
Bärlocher F, Graça MAS (2002) Exotic riparian vegetation lowers fungal diversity but not leaf decomposition in Portuguese streams. Freshw Biol 47:1123–1135. https://doi.org/10.1046/j.1365-2427.2002.00836.x
Menéndez M, Descals E, Riera T, Moya O (2013) Do non-native Platanus hybrida riparian plantations affect leaf litter decomposition in streams? Hydrobiologia 716:5–20. https://doi.org/10.1007/s10750-013-1539-0
Ferreira V, Faustino H, Raposeiro PM, Gonçalves V (2017) Replacement of native forests by conifer plantations affects fungal decomposer community structure but not litter decomposition in Atlantic island streams. For Ecol Manage 389:323–330. https://doi.org/10.1016/j.foreco.2017.01.004
Gulis V, Suberkropp K (2003) Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshw Biol 48:123–134. https://doi.org/10.1046/j.1365-2427.2003.00985.x
Ferreira V, Castagneyrol B, Koricheva J, Gulis V, Chauvet E, Graça MAS (2015) A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biol Rev 90:669–688. https://doi.org/10.1111/brv.12125
Woodward G, Gessner MO, Giller PS, Gulis V, Hladyz S, Lecerf A, Malmqvist B, McKie BG, Tiegs SD, Cariss H, Dobson M, Elosegi A, Ferreira V, Graça MAS, Fleituch T, Lacoursière JO, Nistorescu M, Pozo J, Risnoveanu G, Schindler M, Vadineanu A, Vought LB-M, Chauvet E (2012) Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336:1438–1440. https://doi.org/10.1126/science.1219534
Rosemond AD, Benstead JP, Bumpers PM, Gulis V, Kominoski JS, Manning DWP, Suberkropp K, Wallace JB (2015) Experimental nutrient additions accelerate terrestrial carbon loss from stream ecosystems. Science 347:1142–1145. https://doi.org/10.1126/science.aaa1958
Pereira A, Trabulo J, Fernandes I, Pascoal C, Cássio F, Duarte S (2017) Spring stimulates leaf decomposition in moderately eutrophic streams. Aquat Sci 79:197–207. https://doi.org/10.1007/s00027-016-0490-3
Pereira A, Ferreira V (2021) Invasion of native riparian forests by Acacia species affects in-stream litter decomposition and associated microbial decomposers. Microb Ecol 81:14–25. https://doi.org/10.1007/s00248-020-01552-3
Lowe SR, Woodford DJ, Impson DN, Day JA (2008) The impact of invasive fish and invasive riparian plants on the invertebrate fauna of the Rondegat River, Cape Floristic Region, South Africa. African J Aquat Sci 33:51–62. https://doi.org/10.2989/AJAS.2007.33.1.6.390
Railoun MZ (2018) Impacts of the invasive tree Acacia mearnsii on riparian and instream aquatic environments in the Cape Floristic Region, South Africa. Dissertation, Stellenbosch University
Graça MAS, Poquet JM (2014) Do climate and soil influence phenotypic variability in leaf litter, microbial decomposition and shredder consumption? Oecologia 174:1021–1032. https://doi.org/10.1007/s00442-013-2825-2
Ferreira V, Raposeiro PM, Pereira A, Cruz AM, Costa AC, Graça MAS, Gonçalves V (2016) Leaf litter decomposition in remote oceanic island streams is driven by microbes and depends on litter quality and environmental conditions. Freshw Biol 61:783–799. https://doi.org/10.1111/fwb.12749
Bärlocher F, Gessner MO, Graça MAS (2020) Methods to study litter decomposition. A practical guide. Springer, Dordrecht
Goering HK, Van Soest PJ (1970) Forage fiber analyses (apparatus, reagents, procedures, and some applications). Agricultural Research Service, Washington DC
Gessner MO, Chauvet E (1993) Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl Environ Microbiol 59:502–507
Zar JH (1999) Biostatistical analysis. Prentice-Hall, Englewood Cliffs
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Gulis V, Ferreira V, Graça MAS (2006) Stimulation of leaf litter decomposition and associated fungi and invertebrates by moderate eutrophication: implications for stream assessment. Freshw Biol 51:1655–1669. https://doi.org/10.1111/j.1365-2427.2006.01615.x
Huryn AD, Butz Huryn VM, Arbuckle CJ, Tsomides L (2002) Catchment land-use, macroinvertebrates and detritus processing in headwater streams: taxonomic richness versus function. Freshw Biol 47:401–415. https://doi.org/10.1046/j.1365-2427.2002.00812.x
Fernandes I, Seena S, Pascoal C, Cássio F (2014) Elevated temperature may intensify the positive effects of nutrients on microbial decomposition in streams. Freshw Biol 59:2390–2399. https://doi.org/10.1111/fwb.12445
Atwood TB, Wiegner TN, Turner P, MacKenzie RA (2010) Potential effects of an invasive nitrogen-fixing tree on a Hawaiian stream food web. Pacific Sci 64:367–379. https://doi.org/10.2984/64.3.367
Mineau MM, Baxter CV, Marcarelli AM (2011) A non-native riparian tree (Elaeagnus angustifolia) changes nutrient dynamics in streams. Ecosystems 14:353–365. https://doi.org/10.1007/s10021-011-9415-0
Ferreira V, Chauvet E (2011) Synergistic effects of water temperature and dissolved nutrients on litter decomposition and associated fungi. Glob Chang Biol 17:551–564. https://doi.org/10.1111/j.1365-2486.2010.02185.x
Manning DWP, Rosemond AD, Gulis V, Benstead JP, Kominoski JS (2018) Nutrients and temperature additively increase stream microbial respiration. Glob Chang Biol 24:e233–e247. https://doi.org/10.1111/gcb.13906
Ferreira V, Chauvet E, Canhoto C (2015) Effects of experimental warming, litter species, and presence of macroinvertebrates on litter decomposition and associated decomposers in a temperate mountain stream. Can J Fish Aquat Sci 72:206–216. https://doi.org/10.1139/cjfas-2014-0119
Ferreira V, Canhoto C (2015) Future increase in temperature may stimulate litter decomposition in temperate mountain streams: evidence from a stream manipulation experiment. Freshw Biol 60:881–892. https://doi.org/10.1111/fwb.12539
Bärlocher F, Stewart M, Ryder D (2012) Processing of Eucalyptus viminalis leaves in Australian streams—importance of aquatic hyphomycetes and zoosporic fungi. Fundam Appl Limnol 179:305–319. https://doi.org/10.1127/1863-9135/2012/0229
Baldy V, Gessner MO, Chauvet E (1995) Bacteria, fungi and the breakdown of leaf litter in a large river. Oikos 74:93–102. https://doi.org/10.2307/3545678
Marks JC (2019) Revisiting the fates of dead leaves that fall into streams. Annu Rev Ecol Evol Syst 50:547–568. https://doi.org/10.1146/annurev-ecolsys-110218-024755
Wang F, Lin D, Li W, Dou P, Han L, Huang M, Qian S, Yao J (2020) Meiofauna promotes litter decomposition in stream ecosystems depending on leaf species. Ecol Evol 10:9257–9270. https://doi.org/10.1002/ece3.6610
Gessner MO, Chauvet E (1994) Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75:1807–1817. https://doi.org/10.2307/1939639
Lecerf A, Chauvet E (2008) Intraspecific variability in leaf traits strongly affects alder leaf decomposition in a stream. Basic Appl Ecol 9:598–605. https://doi.org/10.1016/j.baae.2007.11.003
Fernandes I, Pascoal C, Guimarães H, Pinto R, Sousa I, Cássio F (2012) Higher temperature reduces the effects of litter quality on decomposition by aquatic fungi. Freshw Biol 57:2306–2317. https://doi.org/10.1111/fwb.12004
Zhang M, Cheng X, Geng Q, Shi Z, Luo Y, Xu X (2019) Leaf litter traits predominantly control litter decomposition in streams worldwide. Glob Ecol Biogeogr 28:1469–1486. https://doi.org/10.1111/geb.12966
Li AOY, Ng LCY, Dudgeon D (2009) Effects of leaf toughness and nitrogen content on litter breakdown and macroinvertebrates in a tropical stream. Aquat Sci 71:80–93. https://doi.org/10.1007/s00027-008-8117-y
Ferreira V, Encalada AC, Graça MAS (2012) Effects of litter diversity on decomposition and biological colonization of submerged litter in temperate and tropical streams. Freshw Sci 31:945–962. https://doi.org/10.1899/11-062.1
Pereira A, Geraldes P, Lima-Fernandes E, Fernandes I, Cássio F, Pascoal C (2016) Structural and functional measures of leaf-associated invertebrates and fungi as predictors of stream eutrophication. Ecol Indic 69:648–656. https://doi.org/10.1016/j.ecolind.2016.05.017
Campbell IANC, James KIMR, Hart BT, Devereaux A (1992) Allochthonous coarse particulate organic material in forest and pasture reaches of two south-eastern Australian streams. Freshw Biol 27:353–365. https://doi.org/10.1111/j.1365-2427.1992.tb00545.x
Raposeiro PM, Martins GM, Moniz I, Cunha A, Costa AC, Gonçalves V (2014) Leaf litter decomposition in remote oceanic islands: the role of macroinvertebrates vs. microbial decomposition of native vs. exotic plant species. Limnologica 45:80–87. https://doi.org/10.1016/j.limno.2013.10.006
Raposeiro PM, Ferreira V, Gea G, Gonçalves V (2018) Contribution of aquatic shredders to leaf litter decomposition in Atlantic island streams depends on shredder density and litter quality. Mar Freshw Res 69:1432–1439. https://doi.org/10.1071/MF18020
Graça MAS, Cressa C (2010) Leaf quality of some tropical and temperate tree species as food resource for stream shredders. Int Rev Hydrobiol 95:27–41. https://doi.org/10.1002/iroh.200911173
Ferreira V, Elosegi A, Gulis V, Pozo J, Graça MAS (2006) Eucalyptus plantations affect fungal communities associated with leaf-litter decomposition in Iberian streams. Arch Hydrobiol 166:467–490. https://doi.org/10.1127/0003-9136/2006/0166-0467
Arroita M, Aristi I, Flores L, Larrañaga A, Díez J, Mora J, Romaní AM, Elosegi A (2012) The use of wooden sticks to assess stream ecosystem functioning: comparison with leaf breakdown rates. Sci Total Environ 440:115–122. https://doi.org/10.1016/j.scitotenv.2012.07.090
Yeung ACY, Kreutzweiser DP, Richardson JS (2019) Stronger effects of litter origin on the processing of conifer than broadleaf leaves: a test of home-field advantage of stream litter breakdown. Freshw Biol 64:1755–1768. https://doi.org/10.1111/fwb.13367
Artigas J, Romaní AM, Sabater S (2008) Effect of nutrients on the sporulation and diversity of aquatic hyphomycetes on submerged substrata in a Mediterranean stream. Aquat Bot 88:32–38. https://doi.org/10.1016/j.aquabot.2007.08.005
Geraldes P (2011) Fungal communities and functional measures as indicators of stream ecosystem health. Dissertation, Minho University
Noel L, Bärlocher F, Culp JM, Seena S (2016) Nutrient enrichment and flow regulation impair structure and function of a large river as revealed by aquatic hyphomycete species richness, biomass, and decomposition rates. Freshw Sci 35:1148–1163. https://doi.org/10.1086/689180
Arsuffi TL, Suberkropp K (1984) Leaf processing capabilities of aquatic hyphomycetes—interspecific differences and influence on shredder feeding preferences. Oikos 42:144–154. https://doi.org/10.2307/3544786
Arsuffi TL, Suberkropp K (1988) Effects of fungal mycelia and enzymatically degraded leaves on feeding and performance of caddisfly (Trichoptera) larvae. J North Am Benthol Soc 7:205–211. https://doi.org/10.2307/1467420
Brosed M, Jabiol J, Gessner MO (2017) Nutrient stoichiometry of aquatic hyphomycetes: interstrain variation and ergosterol conversion factors. Fungal Ecol 29:96–102. https://doi.org/10.1016/j.funeco.2017.04.008
Kominoski JS, Shah JJF, Canhoto C, Fischer DG, Giling DP, González E, Griffiths NA, Larrañaga A, LeRoy CJ, Mineau MM, McElarney YR, Shirley SM, Swan CM, Tiegs SD (2013) Forecasting functional implications of global changes in riparian plant communities. Front Ecol Environ 11:423–432. https://doi.org/10.1890/120056
Figueiredo A (2016) The contribution of network driving factors to explain invasion patterns: the role of water on Madeira Island. Water Territories, 2nd International Congress of CEGOT, 7-9 March, Faculdade De Letras Da Universidade De Coimbra, Coimbra, Portugal: 109-118
Acknowledgements
We thank Olímpia Sobral (CFE, University of Coimbra) for all the help in the field and the laboratory, and Teresa Gonçalves and Chantal Fernandes (CNC, University of Coimbra) for the use of the lyophilizer. Water nutrient analyses were ordered to Centro de Apoio Científico-Tecnolóxico á Investigación (CACTI, University of Vigo, Spain). Ergosterol analyses were ordered to Instituto do Ambiente Tecnologia e Vida (IATV, University of Coimbra, Portugal).
Availability of Data and Materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Funding
This study was financed by the Portuguese Foundation for Science and Technology (FCT) through the exploratory project IF/00129/2014 granted to Verónica Ferreira and through the strategic project UIDP/04292/2020 granted to MARE. Ana Pereira received a doctoral fellowship from FCT (SFRH/BD/118069/2016), financed by the European Social Fund (FSE) from the European Union (UE), through the Programa Operacional Regional Centro (CENTRO 2020) and by National Funds from the Orçamento de Estado, through Ministério da Ciência, Tecnologia e Ensino Superior (MCTES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Supplementary Information
ESM 1
(PDF 361 kb)
Rights and permissions
About this article
Cite this article
Pereira, A., Figueiredo, A. & Ferreira, V. Invasive Acacia Tree Species Affect Instream Litter Decomposition Through Changes in Water Nitrogen Concentration and Litter Characteristics. Microb Ecol 82, 257–273 (2021). https://doi.org/10.1007/s00248-021-01749-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-021-01749-0