Abstract
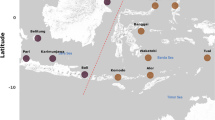
Assessing microbial identity, diversity, and community structure could be a valuable tool for monitoring the impact of xenobiotics and anthropogenic inputs in rivers, especially in urban and industrial settings. Here, we characterize the Nile River microbial community in water and sediments in summer and winter at five locations that span its natural flow through the Cairo metropolis. 16S rRNA gene datasets were analyzed to identify the role played by sample type (sediment versus water), season, and location in shaping the community, as well as to predict functional potential of the Nile River microbiome. Microbial communities were mostly influenced by sampling type (sediments versus water), while seasonal effects were only observed in water samples. Spatial differences did not represent a significant factor in shaping the community in either summer or winter seasons. Proteobacteria was the most abundant phylum in both water and sediment samples, with the order Betaproteobacteriales being the abundant one. Chloroflexi and Bacteroidetes were also prevalent in sediment samples, while Cyanobacteria and Actinobacteria were abundant in water samples. The linear discriminative analysis effect size (LEfSe) identified the cyanobacterial genus Cyanobium PCC-6307 as the main variable between summer and winter water. Sequences representing human and animal potential pathogens, as well as toxin-producing Cyanobacteria, were identified in low abundance within the Nile microbiome. Functionally predicted metabolic pathways predicted the presence of antibiotic biosynthesis, as well as aerobic xenobiotic degradation pathways in the river microbiome.






Similar content being viewed by others
References
Asit KB (1970) History of hydrology. North Holland Publishing Company, Amsterdam
Khatri N, Tyagi S (2015) Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front Life Sci 8(1):23–39
Fan L, Song C, Meng S, Qiu L, Zheng Y, Wu W, Qu J, Li D, Zhang C, Hu G (2016) Spatial distribution of planktonic bacterial and archaeal communities in the upper section of the tidal reach in Yangtze River. Sci Rep 6:39147
Linz AM, Crary BC, Shade A, Owens S, Gilbert JA, Knight R, McMahon KD (2017) Bacterial community composition and dynamics spanning five years in freshwater bog lakes. mSphere 2(3):e00169–e00117
Morrison JM, Baker KD, Zamor RM, Nikolai S, Elshahed MS, Youssef NH (2017) Spatiotemporal analysis of microbial community dynamics during seasonal stratification events in a freshwater lake (Grand Lake, OK, USA). PLoS One 12(5):e0177488
Staley C, Gould TJ, Wang P, Phillips J, Cotner JB, Sadowsky MJ (2015) Species sorting and seasonal dynamics primarily shape bacterial communities in the Upper Mississippi River. Sci Total Environ 505:435–445
Youssef NH, Ashlock-Savage KN, Elshahed MS (2012) Phylogenetic diversities and community structure of members of the extremely halophilic Archaea (order Halobacteriales) in multiple saline sediment habitats. Appl Environ Microbiol 78(5):1332–1344
Ali E, Shabaan-Dessouki S, Soliman A, El Shenawy A (2014) Characterization of chemical water quality in the Nile River, Egypt. Int J Pure Appl Biosci 2(3):35–53
Badr E-SA, El-Sonbati MA (1772) Nassef HM (2013) Water quality assessment in the Nile River, Damietta branch, Egypt. Catrina 283:1–23
Sutcliffe J, Hurst S, Awadallah AG, Brown E, Hamed K (2016) Harold Edwin Hurst: the Nile and Egypt, past and future. Hydrol Sci J 61(9):1557–1570
Fielding L, Najman Y, Millar I, Butterworth P, Garzanti E, Vezzoli G, Barfod D, Kneller B (2018) The initiation and evolution of the River Nile. Earth Planet Sci Lett 489:166–178
Mellander P-E, Gebrehiwot SG, Gärdenäs AI, Bewket W, Bishop K (2013) Summer rains and dry seasons in the Upper Blue Nile Basin: the predictability of half a century of past and future spatiotemporal patterns. PLoS One 8(7):e68461
Abu-Lughod JL, AlSayyad N (2017) Encyclopædia Britannica, inc. Encyclopædia Britannica [Internet]. https://www.britannica.com/place/Cairo. Accessed 1 Jul 2020
El-Sheekh M (2009) River Nile pollutants and their effect on life forms and water quality. In: Dumont HJ (eds) The Nile. Monographiae Biologicae, vol 89. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-9726-3_19
El Gohary R (2015) Agriculture, industry, and wastewater in the Nile Delta. IJSRAS 2:159–172
Abdel-Satar AM, Ali MH, Goher ME (2017) Indices of water quality and metal pollution of Nile River, Egypt. Egypt J Aquat Res 43(1):21–29
Rennie HG (2020) Water management in New Zealand's Canterbury Region: a sustainability framework Bryan R. Jenkins. Springer, Dordrecht, 2018, pp 524. N Z Geog 76:162–163. https://doi.org/10.1111/nzg.12272
Eraqi WA, ElRakaiby MT, Megahed SA, Yousef NH, Elshahed MS, Yassin AS (2018) The Nile River microbiome reveals a remarkably stable community between wet and dry seasons, and sampling sites, in a large urban metropolis (Cairo, Egypt). Omics 22(8):553–564
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, DC
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6(8):1621–1624
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41(D1):D590–D596
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Chao A, Lee S-M (1992) Estimating the number of classes via sample coverage. J Am Stat Assoc 87(417):210–217
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423 623--656
Simpson EH (1949) Measurement of diversity. Nature 163(4148):688
Dhariwal A, Chong J, Habib S, King IL, Agellon LB, Xia J (2017) MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res 45(W1):W180–W188
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60
Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Thurber RLV, Knight R (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31(9):814–821
McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P (2012) An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6(3):610–618
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27(16):2194–2200
Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M (2013) Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res 42(D1):D199–D205
Kanehisa M, Goto S (2000) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28(1):27–30
Good IJ (1953) The population frequencies of species and the estimation of population parameters. Biometrika 40(3-4):237–264
Koizumi Y, Kojima H, Oguri K, Kitazato H, Fukui M (2004) Vertical and temporal shifts in microbial communities in the water column and sediment of saline meromictic Lake Kaiike (Japan), as determined by a 16S rDNA-based analysis, and related to physicochemical gradients. Environ Microbiol 6(6):622–637
Yoshimura KM (2019) Influences of particulate matter transport, export, and sedimentation on microbial community ecology. Yoshimura, Kristin Michelle. University of Delaware, ProQuest Dissertations Publishing, 2019. 13859788
Abia ALK, James C, Ubomba-Jaswa E, Benteke Momba MN (2017) Microbial remobilisation on riverbed sediment disturbance in experimental flumes and a human-impacted river: implication for water resource management and public health in developing sub-Saharan African countries. Int J Environ Res Public Health 14(3):306
Giannuzzi L (2018) Cyanobacteria growth kinetics. Algae, Yee Keung Wong, IntechOpen. https://doi.org/10.5772/intechopen.81545
Martinez-Garcia M, Brazel DM, Swan BK, Arnosti C, Chain PS, Reitenga KG, Xie G, Poulton NJ, Gomez ML, Masland DE (2012) Capturing single cell genomes of active polysaccharide degraders: an unexpected contribution of Verrucomicrobia. PLoS One 7(4):e35314
Sichert A, Corzett CH, Schechter MS, Unfried F, Markert S, Becher D, Fernandez-Guerra A, Liebeke M, Schweder T, Polz MF (2020) Verrucomicrobia use hundreds of enzymes to digest the algal polysaccharide fucoidan. Nat Microbiol 5:1026–1039. https://doi.org/10.1038/s41564-020-0720-2
Casadevall A, L-a P (2014) Microbiology: ditch the term pathogen. Nature News 516(7530):165–166
Anudit C, Kooltheat N, Potup P, Sranujit RP, Usuwanthim K (2016) Nosocomial infection of multidrug-resistant Acinetobacter baumannii in Thailand. Am J Infect Control 44(10):1161–1163
Joly-Guillou ML (2005) Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect 11(11):868–873
Garnacho-Montero J, Amaya-Villar R (2010) Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis 23(4):332–339
Ramadan N (2016) Prevalence of legionella among pneumonia patients and environmental water samples in an Egyptian University Hospital. Intern Arabic J Antimicrob Agents 6(2)
El-Liethya MA, Hemdana BA, El-Shatouryb EH, Abou-Zeidb MA, Samhana FA, El-Taweela GE (2016) Prevalence of Legionella spp. and Helicobacter pylori in different water resources in Egypt. Egyptian Journal of Environmental Research 4
Loeffelholz MJ (2014) Respiratory infections, an issue of clinics in laboratory medicine, vol 34-2, 1st edn. University of Texas Medical Branch Galveston, Texas. Imprint: Elsevier. Published Date: 3rd June 2014
Albarral V, Sanglas A, Palau M, Miñana-Galbis D, Fusté MC (2016) Potential pathogenicity of Aeromonas hydrophila complex strains isolated from clinical, food, and environmental sources. Can J Microbiol 62(4):296–306
Austin B, Austin DA (2016) Bacterial fish pathogens: disease of farmed and wild fish, 6th ed. Springer International Publishing, p 732. https://doi.org/10.1007/978-3-319-32674-0
Rogalla D, Bomar PA (2020) Listeria Monocytogenes. [Updated 2020 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534838/
WHO (2018) Listeriosis: fact sheet. Geneva: World Health Organization, 2018. http://www.who.int/mediacentre/factsheets/listeriosis/en/ (accessed 20 February 2018)
de Noordhout CM, Devleesschauwer B, Angulo FJ, Verbeke G, Haagsma J, Kirk M, Havelaar A, Speybroeck N (2014) The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis 14(11):1073–1082
Gad MA, Al-Herrawy AZ (2016) Real-time PCR detection of Acanthamoeba species in the Egyptian aquatic environment. Int J Pharma Clin Res 8:1510–1515
Rastogi RP, Madamwar D, Incharoensakdi A (2015) Bloom dynamics of cyanobacteria and their toxins: environmental health impacts and mitigation strategies. Front Microbiol 6:1254
Kindaichi T, Yuri S, Ozaki N, Ohashi A (2012) Ecophysiological role and function of uncultured Chloroflexi in an anammox reactor. Water Sci Technol 66(12):2556–2561
Florou-Paneri P, Christaki E, Bonos E (2013) Lactic acid bacteria as source of functional ingredients. In: Marcelino Kongo J (eds.) Lactic acid bacteria - R & D for food, health and livestock purposes. https://doi.org/10.5772/47766
Acknowledgments
The authors are deeply grateful to Prof. Ramy Karam Aziz, Chairperson of the Department of Microbiology and Immunology, Faculty of Pharmacy, Cairo University, for providing critical insights and valuable feedback that greatly assisted the research.
Funding
This work has been partly supported by NSF-DEB grant 2016423 to NHY and MSE and by the Academy of Scientific Research and Technology (ASRT) JESOR Project #3046 to MSE and ASY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no competing interests of any sort.
Rights and permissions
About this article
Cite this article
Eraqi, W.A., ElRakaiby, M.T., Megahed, S.A. et al. Spatiotemporal Analysis of the Water and Sediment Nile Microbial Community Along an Urban Metropolis. Microb Ecol 82, 288–298 (2021). https://doi.org/10.1007/s00248-020-01674-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-020-01674-8