Abstract
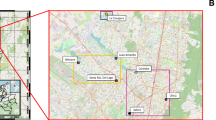
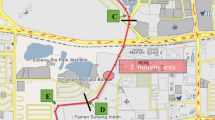
Microbial communities in surface waters used for recreational purposes are indicators of contamination and risk of contact with human pathogens. Hence, monitoring microbial communities in recreational waters is important for potential public health threats to humans. Such monitoring is rare in Colombia, even in its capital, Bogotá, the most populous city in the country. This city encompasses metropolitan and linear parks with recreational water bodies that are used frequently by the public, and the presence of pathogens can compromise the health of the citizens. Therefore, we examined the bacterial, and eukaryotic communities in urban recreational lakes (URL) in four metropolitan parks in Bogotá, Colombia. Samples from four metropolitan parks (Los Novios, Simon Bolivar, El Tunal, and Timiza) and one stream contaminated with sewage from a linear park (El Virrey) were collected. We used amplicon next-generation sequencing of the 16S-rRNA gene and 18S-rRNA gene to characterize microbial communities followed by bioinformatics analyses. In addition, general water quality parameters—pH, hardness, acidity, alkalinity, dissolved oxygen, and nitrites—were recorded using a commercial kit. Genera of pathogens, including Legionella, Pseudomonas, Mycobacterium, Candida, and Naegleria, were found in lake waters. The stream El Virrey was, however, the only surface water that showed an abundance of fecal bacteria, often associated with low oxygen concentrations. All water bodies showed a predominance of fungal phyla, except for the lake at Timiza. This lake showed the highest pH, and its ecological dynamics are likely different from other water bodies. Likewise, some URLs displayed a greater abundance of cyanobacteria, including toxin-producing species. Algal genera associated with eutrophication were predominant among primary producing microorganisms. This study shows for the first time the description of the bacterial and eukaryotic communities of some URLs and a stream in Bogotá. The URLs and the stream harbored various pathogens that might pose a risk to the citizen’s health.





Similar content being viewed by others
Data Availability
The datasets generated and/or analyzed during the current study will be available in the The European Nucleotide Archive (ENA) repository. Study accession number: PRJEB38738.
Abbreviations
- MC:
-
Microbial communities
- URL:
-
Urban recreational lake
- NGS:
-
Next generation sequencing
- ASV:
-
Amplicon sequence variant
- NTM:
-
Nontuberculous mycobacteria
References
Navia SLÁ de, Torres SME (2013) Calidad sanitaria del agua del Parque Natural Chicaque. NOVA 11:45–51. https://doi.org/10.22490/24629448.1026
World Health Organization (2003) WHO Guidelines for safe recreational water environments - vol 1, Coastal and Fresh Waters. https://www.who.int/water_sanitation_health/publications/srwe1/en/
Postel S, Carpenter S (1997) In: Daily GC (ed) Freshwater ecosystme services, vol 195. Island Press, Washington, DC, pp 195–216
Garcia X, Barceló D, Comas J, Corominas L, Hadjimichael A, Page TJ, Acuña V (2016) Placing ecosystem services at the heart of urban water systems management. Sci Total Environ 563–564:1078–1085. https://doi.org/10.1016/j.scitotenv.2016.05.010
Polat AT, Akay A (2015) Relationships between the visual preferences of urban recreation area users and various landscape design elements. Urban For Urban Green 14:573–582. https://doi.org/10.1016/j.ufug.2015.05.009
Clinton S, Johnson J, Lambirth K, Sun S, Brouwer C, Keen O, Redmond M, Fodor A, Gibas C (2020) Sediment microbial diversity in urban Piedmont North Carolina watersheds receiving wastewater input. Water J 12:1557. https://doi.org/10.3390/w12061557
Gerba CP, Smith JE (2005) Sources of pathogenic microorganisms and their fate during land application of wastes. J Environ Qual 34:42–48. https://doi.org/10.2134/jeq2005.0042a
Goss M, Richards C (2008) Development of a risk-based index for source water protection planning, which supports the reduction of pathogens from agricultural activity entering water resources. J Environ Manag 87:623–632. https://doi.org/10.1016/j.jenvman.2006.12.048
Chu BTT, Petrovich ML, Chaudhary A, Wright D, Murphy B, Wells G, Poretsky R (2018) Metagenomics reveals the impact of wastewater treatment plants on the dispersal of microorganisms and genes in aquatic sediments. Appl Environ Microbiol. https://doi.org/10.1128/AEM.02168-17
Ashbolt N, Grabow W, Snozzi M (2001) Indicators of microbial water quality. In: Fewtrell L, Bartram J (eds) Water quality- guidelines, standards and health, vol 1, pp 289–316. https://apps.who.int/iris/handle/10665/42442
World Health Organization (2018) WHO recommendations on scientific, analytical and epidemiological developments relevant to the parameters for bathing water quality in the Bathing Water Directive (2006/7/EC). https://www.who.int/water_sanitation_health/publications/who-recommendations-to-european-water-directive/en/
Hlavsa MC, Roberts VA, Kahler AM, Hilborn ED, Mecher TR, Beach MJ, Wade TJ, Yoder JS (2015) Outbreaks of illness associated with recreational water — United States, 2011–2012. MMWR Morb Mortal Wkly Rep 64:668–672
Rodrigues C, Cunha MÂ (2017) Assessment of the microbiological quality of recreational waters: indicators and methods. Euro-Mediterr J Environ Integr 2:25. https://doi.org/10.1007/s41207-017-0035-8
Schets FM, van Wijnen JH, Schijven JF, Schoon H, de Roda Husman AM (2008) Monitoring of waterborne pathogens in surface waters in Amsterdam, the Netherlands, and the potential health risk associated with exposure to Cryptosporidium and Giardia in these waters. Appl Environ Microbiol 2069–2078:74. https://doi.org/10.1128/AEM.01609-07
Pilotto L, Hobson P, Burch MD, Ranmuthugala G, Attewell R, Weightman W (2004) Acute skin irritant effects of cyanobacteria (blue-green algae) in healthy volunteers. Aust N Z J Public Health 28:220–224. https://doi.org/10.1111/j.1467-842X.2004.tb00699.x
Liu X, Hou W, Dong H, Wang S, Jiang H, Wu G, Yang J, Li G (2016) Distribution and diversity of cyanobacteria and eukaryotic algae in Qinghai–Tibetan Lakes. Geomicrobiol J 33:860–869. https://doi.org/10.1080/01490451.2015.1120368
Bandh SA, Kamili AN, Ganai BA, Lone BA (2016) Opportunistic fungi in lake water and fungal infections in associated human population in Dal Lake, Kashmir. Microb Pathog 93:105–110. https://doi.org/10.1016/j.micpath.2016.01.022
Wurts W Durborow R (1992) Interactions of pH, carbon dioxide, alkalinity and hardness in fish ponds. Iowa State Univ Sci Technol. https://www.ncrac.org/content/interactions-ph-carbon-dioxide-alkalinity-and-hardness-fish-ponds
Lindström ES, Agterveld MPK-V, Zwart G (2005) Distribution of typical freshwater bacterial groups is associated with pH, temperature, and lake water retention time. Appl Environ Microbiol 71:8201–8206. https://doi.org/10.1128/AEM.71.12.8201-8206.2005
Sangolkar LN, Maske SS, Chakrabarti T (2006) Methods for determining microcystins (peptide hepatotoxins) and microcystin-producing cyanobacteria. Water Res 40:3485–3496. https://doi.org/10.1016/j.watres.2006.08.010
Ozawa K, Fujioka H, Muranaka M, Yokoyama A, Katagami Y, Homma T, Ishikawa K, Tsujimura S, Kumagai M, Watanabe MF et al (2005) Spatial distribution and temporal variation of Microcystis species composition and microcystin concentration in Lake Biwa. Environ Toxicol 20:270–276. https://doi.org/10.1002/tox.20117
Harke MJ, Steffen MM, Gobler CJ, Otten TG, Wilhelm SW, Wood SA, Paerl HW (2016) A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54:4–20. https://doi.org/10.1016/j.hal.2015.12.007
Komárek J (2002) Problems in cyanobacterial taxonomy: implication for most common toxin producing species. Rapporti Istisan, pp 6–43
Barnes AN, Anderson JD, Mumma J, Mahmud ZH, Cumming O (2018) The association between domestic animal presence and ownership and household drinking water contamination among peri-urban communities of Kisumu, Kenya. PLoS ONE 13:e0197587. https://doi.org/10.1371/journal.pone.0197587
Tan JS (1933–1943) Human zoonotic infections transmitted by dogs and cats. Arch Intern Med 1997:157. https://doi.org/10.1001/archinte.1997.00440380035003
Schijven J, de Roda Husman AM (2006) A survey of diving behavior and accidental water ingestion among Dutch occupational and sport divers to assess the risk of infection with waterborne pathogenic microorganisms. Environ Health Perspect 114:712–717. https://doi.org/10.1289/ehp.8523
Hlavsa MC, Roberts VA, Kahler AM, Hilborn ED, Wade TJ, Backer LC, Yoder JS (2014) Recreational water–associated disease outbreaks — United States, 2009–2010. MMWR Morb Mortal Wkly Rep 63:6–10
Sanborn M, Takaro T (2013) Recreational water–related illness. Can. Fam. Physician 59:491–495
Duy TN, Lam PKS, Shaw GR, Connell DW (2000) Toxicology and risk assessment of freshwater cyanobacterial (Blue-Green Algal) toxins in water. In: Ware GW (ed) Reviews of environmental contamination and toxicology: continuation of residue reviews.Springer, New York, pp 113–185
Wilson M, Chapman O, Mendenhall W, Bennet H, Ezekiel M, Baker J, Eliot C, Gray L (1935) Certain aspects of land problems and government land policies, part VII of the report on land planning; 1, vol 24. 1st edn. U.S. Government Printing Office, Washington, DC
Cahill S, Llimona F, Gràcia J (2003) Spacing and nocturnal activity of wild boar Sus scrofa in a Mediterranean metropolitan park. Wildlife Biol 9:3–13. https://doi.org/10.2981/wlb.2003.058
McDonald SM, Price GG (2009) Addressing declining metropolitan park use: a case study of Melbourne, Victoria, Australia. Managing Leisure 14:28–37. https://doi.org/10.1080/13606710802551239
Kullmann K (2011) Thin parks / thick edges: towards a linear park typology for (post)infrastructural sites. J Landsc Ecol 6:70–81. https://doi.org/10.1080/18626033.2011.9723456
Mugavin D (2004) Adelaide’s greenway: river Torrens Linear Park. Landscape Urban Plan 68:223–240. https://doi.org/10.1016/j.landurbplan.2003.07.002
Crewe K (2001) Linear parks and urban neighbourhoods: a study of the crime impact of the Boston south-west corridor. J Urban Des 6:245–264. https://doi.org/10.1080/13574800120105779
Instituto Distrital de Recreacion y Deporte (IDRD) Parque Metropolitano Simón Bolívar. https://www.idrd.gov.co/parque-metropolitano-simon-bolivar. Accessed 28 Jul 2020
Instituto Distrital de Recreacion y Deporte (IDRD) Parque El Tunal. https://www.idrd.gov.co/parque-tunal-0. Accessed 28 Jul 2020
Instituto Distrital de Recreación y Deporte (IDRD) Parque Timiza. https://www.idrd.gov.co/parque-timiza. Accessed 28 Jul 2020
Moriarty EM, Karki N, Mackenzie M, Sinton LW, Wood DR, Gilpin BJ (2011) Faecal indicators and pathogens in selected New Zealand waterfowl. New Zeal J Mar Fresh 45:679–688. https://doi.org/10.1080/00288330.2011.578653
Nguyen KH, Senay C, Young S, Nayak B, Lobos A, Conrad J, Harwood VJ (2018) Determination of wild animal sources of fecal indicator bacteria by microbial source tracking (MST) influences regulatory decisions. Water Res 144:424–434. https://doi.org/10.1016/j.watres.2018.07.034
Alcaldia Mayor de Bogota. Ruta Parque Lineal El Virrey. http://www.bogotaturismo.gov.co/sites/default/files/rutas/PORTAFOLIO_PARQUE_EL_VIRREY.pdf. Accessed 28 Jun 2020
Instituto de Hidrología, Meteorología y Estudios Ambientales (IDEAM) Comportamiento mensual precipitación 2019. http://www.ideam.gov.co/web/tiempo-y-clima/comportamiento-mensual-precipitacion?p_p_id=110_INSTANCE_Bxb2LKaoA2TC&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&p_p_col_id=column-1&p_p_col_count=1&_110_INSTANCE_Bxb2LKaoA2TC_redirect=http%3A%2F%2Fwww.ideam.gov.co%2Fweb%2Ftiempo-y-clima%2Fcomportamiento-mensual-precipitacion%2F-%2Fdocument_library_display%2FBxb2LKaoA2TC%2Fview%2F98582800%3F_110_INSTANCE_Bxb2LKaoA2TC_redirect%3Dhttp%253A%252F%252Fwww.ideam.gov.co%252Fweb%252Ftiempo-y-clima%252Fcomportamiento-mensual-precipitacion%253Fp_p_id%253D110_INSTANCE_Bxb2LKaoA2TC%2526p_p_lifecycle%253D0%2526p_p_state%253Dnormal%2526p_p_mode%253Dview%2526p_p_col_id%253Dcolumn-1%2526p_p_col_count%253D1&_110_INSTANCE_Bxb2LKaoA2TC_struts_action=%2Fdocument_library_display%2Fview_file_entry&_110_INSTANCE_Bxb2LKaoA2TC_fileEntryId=98576920&_110_INSTANCE_Bxb2LKaoA2TC_version=1.0. Accessed 22 Oct 2020
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R (2011) Global patterns of 16S rRNA gene diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108:4516. https://doi.org/10.1073/pnas.1000080107
Hosen JD, Febria CM, Crump BC, Palmer MA (2017) Watershed urbanization linked to differences in stream bacterial community composition. Front Microbiol 8:1452. https://doi.org/10.3389/fmicb.2017.01452
Li P, Liang H, Lin W-T, Feng F, Luo L (2015) Microbiota dynamics associated with environmental conditions and potential roles of cellulolytic communities in traditional Chinese cereal starter solid-state fermentation. Appl Environ Microbiol 81:5144–5156
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Callahan BJ (2018) Silva taxonomic training data formatted for DADA2 (Silva version 132) [Data set]. Zenodo. https://doi.org/10.5281/zenodo.1172783
Guillou L, Bachar D, Audic S, Bass D, Berney C, Bittner L, Boutte C, Burgaud G, de Vargas C, Decelle J et al (2012) The Protist ribosomal reference database (PR2): a catalog of unicellular eukaryote small sub-unit rRNA gene sequences with curated taxonomy. Nucleic Acids Res 41:D597–D604. https://doi.org/10.1093/nar/gks1160
O’Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, Rajput B, Robbertse B, Smith-White B, Ako-Adjei D (2016) Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res 44:D733–D745
McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. https://doi.org/10.1371/journal.pone.0061217
World Health Organization (2017) WHO guidelines for drinking-water quality: fourth edition incorporating the first addendum. https://www.who.int/publications/i/item/9789241549950
Feng S, Bootsma M, McLellan SL (2018) Human-associated Lachnospiraceae genetic markers improve detection of fecal pollution sources in urban waters. Appl Environ Microbiol 84:e00309–e00318. https://doi.org/10.1128/AEM.00309-18
Newton RJ, Vandewalle JL, Borchardt MA, Gorelick MH, McLellan SL (2011) Lachnospiraceae and Bacteroidales alternative fecal indicators reveal chronic human sewage contamination in an urban harbor. Appl Environ Microbiol 77:6972–6981. https://doi.org/10.1128/AEM.05480-11
McLellan SL, Huse SM, Mueller-Spitz SR, Andreishcheva EN, Sogin ML (2010) Diversity and population structure of sewage-derived microorganisms in wastewater treatment plant influent. Environ Microbiol 12:378–392. https://doi.org/10.1111/j.1462-2920.2009.02075.x
WHO (2017) World Health Organization Guidelines for Drinking-water Quality: fourth edition incorporating the first addendum. https://www.who.int/publications/i/item/9789241549950
Li X, Salam N, Li J-L, Chen Y-M, Yang Z-W, Han M-X, Mou X, Xiao M, Li W-J (2019) Aestuariivirga litoralis gen. Nov., sp. nov., a proteobacterium isolated from a water sample, and proposal of Aestuariivirgaceae fam. nov. Int J Syst Evol Microbiol 69:299–306. https://doi.org/10.1099/ijsem.0.003087
Sun Y, Wang T, Peng X, Wang P, Lu Y (2016) Bacterial community compositions in sediment polluted by perfluoroalkyl acids (PFAAs) using Illumina high-throughput sequencing. Environ Sci Pollut Res 23:10556–10565. https://doi.org/10.1007/s11356-016-6055-0
Zhang S, Zhou H, Sun C, Hu Z, Wang H (2020) Aequorivita lutea sp. nov., a novel bacterium isolated from the estuarine sediment of the Pearl River in China, and transfer of Vitellibacter todarodis and Vitellibacter aquimaris to the genus Aequorivita as Aequorivita todarodis comb. nov. and Aequorivita aquimaris comb. nov. Int J Syst Evol Microbiol 70:3117–3122. https://doi.org/10.1099/ijsem.0.004139
Morohoshi T, Oi T, Aiso H, Suzuki T, Okura T, Sato S (2018) Biofilm formation and degradation of commercially available biodegradable plastic films by bacterial consortiums in freshwater environments. Microbes Environ. https://doi.org/10.1264/jsme2.ME18033
Frangeul L, Quillardet P, Castets A-M, Humbert J-F, Matthijs HC, Cortez D, Tolonen A, Zhang C-C, Gribaldo S, Kehr J-C et al (2008) Highly plastic genome of microcystis aeruginosa PCC 7806, a ubiquitous toxic freshwater cyanobacterium. BMC Genomics 9:274. https://doi.org/10.1186/1471-2164-9-274
Borowitzka MA, Beardall J, Raven JA (2016) The physiology of microalgae, vol 6. Springer, Cham
Nicholls KH, Wujek DE (2015) Chrysophyceae and phaeothamniophyceae. Freshwater algae of North America. Elsevier, Amsterdam, pp 537–586
Nadal F, Ruiz M, Rodríguez MI, Halac S, Olivera P (2012) Evaluación de la calidad de agua para uso recreativo del embalse San Roque, Córdoba, Argentina. I Encuentro de Investigadores en Formación en Recursos Hídricos Instituto Nacional del Agua (INA). Ezeiza, Buenos Aires
Fuenmayor G, Jonte L, Rosales-Loaiza N, Morales E (2009) Crecimiento de la cianobacteria marina Oscillatoria sp. MOF-06 en relación al pH en cultivos discontinuos. Rev Soc Venez Microbiol 29:21–25
Cattaneo MP, Sardi EML (2013) Evolución de la calidad del agua de la cuenca Matanza-Riachuelo. Ciencia y tecnología, pp 251–278
Truque P (2011) Armonización de los estándares de agua potable en las Américas. Organización de Estados Americanos, Washinton DC, EE. UU
Hu A, Li S, Zhang L, Wang H, Yang J, Luo Z, Rashid A, Chen S, Huang W, Yu C-P (1729–1739) Prokaryotic footprints in urban water ecosystems: a case study of urban landscape ponds in a coastal city, China. Environ Pollut 2018:242. https://doi.org/10.1016/j.envpol.2018.07.097
Wang B, Zheng X, Zhang H, Xiao F, He Z, Yan Q (2020) Keystone taxa of water microbiome respond to environmental quality and predict water contamination. Environ Res 187:109666. https://doi.org/10.1016/j.envres.2020.109666
Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S (2011) A guide to the natural history of freshwater Lake Bacteria. Microbiol Mol Biol Rev 75:14. https://doi.org/10.1128/MMBR.00028-10
Jin D, Kong X, Cui B, Jin S, Xie Y, Wang X, Deng Y (2018) Bacterial communities and potential waterborne pathogens within the typical urban surface waters. Sci Rep 8:13368. https://doi.org/10.1038/s41598-018-31706-w
Zhang J, Yang Y, Zhao L, Li Y, Xie S, Liu Y (2015) Distribution of sediment bacterial and archaeal communities in plateau freshwater lakes. Appl Microbiol Biotechnol 99:3291–3302. https://doi.org/10.1007/s00253-014-6262-x
Kagami M, Miki T, Takimoto G (2014) Mycoloop: chytrids in aquatic food webs. Front Microbiol 5:166. https://doi.org/10.3389/fmicb.2014.00166
Wang Y, Guo X, Zheng P, Zou S, Li G, Gong J (2017) Distinct seasonality of chytrid-dominated benthic fungal communities in the neritic oceans (Bohai Sea and North Yellow Sea). Fungal Ecol 30:55–66. https://doi.org/10.1016/j.funeco.2017.08.008
Pernthaler J (2005) Predation on prokaryotes in the water column and its ecological implications. Nat Rev Microbiol 3:537–546. https://doi.org/10.1038/nrmicro1180
Kagami M, Miki T, Takimoto G (2014) Mycoloop: chytrids in aquatic food webs. Front Microbiol 5:166. https://doi.org/10.3389/fmicb.2014.00166
Sime - Ngando T (2012) Phytoplankton Chytridiomycosis: fungal parasites of phytoplankton and their imprints on the food web dynamics. Front Microbiol. https://doi.org/10.3389/fmicb.2012.00361
Comeau AM, Vincent WF, Bernier L, Lovejoy C (2016) Novel chytrid lineages dominate fungal sequences in diverse marine and freshwater habitats. Sci Rep 6:30120. https://doi.org/10.1038/srep30120
Silva-Bedoya LM, Ramírez-Castrillón M, Osorio-Cadavid E (2014) Yeast diversity associated to sediments and water from two Colombian artificial lakes. Braz J Microbiol 45:135–142
Graczyk TK, Majewska AC, Schwab KJ (2008) The role of birds in dissemination of human waterborne enteropathogens. Trends Parasitol 24:55–59. https://doi.org/10.1016/j.pt.2007.10.007
Alcaldía Mayor de Bogotá D.C. (2016) Evaluación final de contratos de mantenimiento (Piscinas, motobombas y lagos). https://www.idrd.gov.co/sitio/idrd/sites/default/files/imagenes/ctos_de_mantenimiento_con_firma.pdf
Instituto Nacional de Salud. Boletín epidemiológico semanal, semana epidemiológica 42, Morbilidad por Enfermedad Diarreica Aguda. 2019. https://www.ins.gov.co/buscador-eventos/Paginas/Vista-Boletin-Epidemilogico.aspx. Accessed 28 Jul 2020
Cheung MK, Au CH, Chu KH, Kwan HS, Wong CK (2010) Composition and genetic diversity of picoeukaryotes in subtropical coastal waters as revealed by 454 pyrosequencing. ISME J 4:1053–1059. https://doi.org/10.1038/ismej.2010.26
Escartin EF, Lozano JS, Garcia OR, Cliver DO (2002) Potential salmonella transmission from ornamental fountains. (Features) J Environ Health 65:9
Díaz-Solano BH, Esteller MV, Garrido Hoyos SE (2011) Calidad físico-química y microbiólogica del agua en parques acuáticos. Hidrobiologica 21:49–62
Makovcova J, Slany M, Babak V, Slana I, Kralik P (2014) The water environment as a source of potentially pathogenic mycobacteria. J Water Health 12:254–263. https://doi.org/10.2166/wh.2013.102
Tortone CA, Zumarraga MJ, Gioffre A, Oriani DS (2018) Utilization of molecular and conventional methods for the identification of nontuberculous mycobacteria isolated from different water sources. Int J Mycobacteriol 7:53–60.
Bland CS, Ireland JM, Lozano E, Alvarez ME, Primm TP (2005) Mycobacterial ecology of the Rio Grande. Appl Environ Microbiol 71:5719–5727. https://doi.org/10.1128/AEM.71.10.5719-5727.2005
Tan KSW (2004) Blastocystis in humans and animals: new insights using modern methodologies. Vet Parasitol 126:121–144. https://doi.org/10.1016/j.vetpar.2004.09.017
Monapathi ME, Bezuidenhout CC, James Rhode OH (2020) Aquatic yeasts: diversity, characteristics and potential health implications. J Water Health 18:91–105. https://doi.org/10.2166/wh.2020.270
Monapathi ME, Bezuidenhout CC, Rhode OHJ (2017) Water quality and antifungal susceptibility of opportunistic yeast pathogens from rivers. Water Sci Technol 75:1319–1331. https://doi.org/10.2166/wst.2016.580
Assress HA, Selvarajan R, Nyoni H, Ntushelo K, Mamba BB, Msagati TAM (Diversity, co-occurrence and implications of fungal communities in wastewater treatment plants, 2019). Sci Rep 9:14056. https://doi.org/10.1038/s41598-019-50624-z
Turner SA, Butler G (2014) The Candida pathogenic species complex. Cold Spring Harb Perspect Med 4:a019778–a019778. https://doi.org/10.1101/cshperspect.a019778
Cafarchia C, Camarda A, Romito D, Campolo M, Quaglia N, Tullio D, Otranto D (2006) Occurrence of yeasts in cloacae of migratory birds. Mycopathologia 161:229–234
Lopes VR, Ramos V, Martins A, Sousa M, Welker M, Antunes A, Vasconcelos VM (2012) Phylogenetic, chemical and morphological diversity of cyanobacteria from Portuguese temperate estuaries. Mar Environ Res 73:7–16
Amer R, Díez B, El-Shehawy R (2009) Diversity of hepatotoxic cyanobacteria in the Nile Delta, Egypt. J Environ Monitor 11:126–133
Frazão B, Martins R, Vasconcelos V (2010) Are known cyanotoxins involved in the toxicity of picoplanktonic and filamentous North Atlantic marine cyanobacteria? Mar Drugs 8:1908–1919
Kim B-H, Hwang S-J, Park M-H, Kim Y-J (2010) Relationship between cyanobacterial biomass and total microcystin-LR levels in drinking and recreational water. Bull Environ Contam Toxicol 85:457–462. https://doi.org/10.1007/s00128-010-0121-y
Codd GA, Morrison LF, Metcalf JS (2005) Cyanobacterial toxins: risk management for health protection. Toxicol Appl Pharm 203:264–272. https://doi.org/10.1016/j.taap.2004.02.016
Ingrid Chorus JB, Falconer IR, Salas HJ (2000) Health risks caused by freshwater cyanobacteria in recreational waters. J Toxicol Environ Health 3:323–347. https://doi.org/10.1080/109374000436364
Giannuzzi L, Sedan D, Echenique R, Andrinolo D (2011) An acute case of intoxication with cyanobacteria and cyanotoxins in recreational water in Salto Grande Dam, Argentina. Mar Drugs 9:2164–2175. https://doi.org/10.3390/md9112164
Xu Y, Wang G, Yang W, Li R (2010) Dynamics of the water bloom-forming Microcystis and its relationship with physicochemical factors in Lake Xuanwu (China). Environ Sci Pollut Res 17:1581–1590. https://doi.org/10.1007/s11356-010-0345-8
Watanabe MF, Oishi S, Harada K-I, Matsuura K, Kawai H, Suzuki M (1988) Toxins contained in Microcystis species of cyanobacteria (blue-green algae). Toxicon 26:1017–1025. https://doi.org/10.1016/0041-0101(88)90200-0
Osborne NJ, Shaw GR (2008) Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol 8:5. https://doi.org/10.1186/1471-5945-8-5
Bicudo DC, Tremarin PI, Almeida PD, Zorzal-Almeida S, Wengrat S, Faustino SB, Costa LF, Bartozek EC, Rocha AC, Bicudo CE (2016) Ecology and distribution of Aulacoseira species (Bacillariophyta) in tropical reservoirs from Brazil. Diatom Research 31:199–215
May L, Bailey-Watts A, Kirika A (2001) The relationship between Trichocerca pusilla (Jennings), Aulacoseira spp. and water temperature in Loch Leven, Scotland, UK. Hydrobiologia 446:29–34
Paerl HW, Valdes LM, Pinckney JL, Piehler MF, Dyble J, Moisander PH (2003) Phytoplankton photopigments as indicators of estuarine and coastal eutrophication. BioScience 53:953–964. https://doi.org/10.1641/0006-3568(2003)053[0953:PPAIOE]2.0.CO;2
Mäemets A (1983) Rotifers as indicators of lake types in Estonia. Hydrobiologia 104:357–361. https://doi.org/10.1007/BF00045991
Kitner M, Poulícková A (2003) Littoral diatoms as indicators for the eutrophication of shallow lakes. Hydrobiologia 506:519–524. https://doi.org/10.1023/B:HYDR.0000008567.99066.92
Penning WE, Mjelde M, Dudley B, Hellsten S, Hanganu J, Kolada A, van den Berg M, Poikane S, Phillips G, Willby N et al (2008) Classifying aquatic macrophytes as indicators of eutrophication in European lakes. Aquat Ecol 42:237–251. https://doi.org/10.1007/s10452-008-9182-y
Ferreira JG, Andersen JH, Borja A, Bricker SB, Camp J, Cardoso da Silva M, Garcés E, Heiskanen A-S, Humborg C, Ignatiades L et al (2011) Overview of eutrophication indicators to assess environmental status within the European Marine Strategy Framework Directive. Estuar Coast Shelf Sci 93:117–131. https://doi.org/10.1016/j.ecss.2011.03.014
Bec A, Martin-Creuzburg D, von Elert E (2006) Trophic upgrading of autotrophic picoplankton by the heterotrophic nanoflagellate Paraphysomonas sp. Limnol Oceanogr 51:1699–1707
Boenigk J, Pfandl K, Stadler P, Chatzinotas A (2005) High diversity of the ‘Spumella-like’flagellates: an investigation based on the SSU rRNA gene gene sequences of isolates from habitats located in six different geographic regions. Environ Microbiol 7:685–697
Buesing N, Gessner MO (2006) Benthic bacterial and fungal productivity and carbon turnover in a freshwater marsh. Appl Environ Microbiol 72:596–605. https://doi.org/10.1128/AEM.72.1.596-605.2006
Peng F-J, Pan C-G, Zhang N-S, ter Braak CJF, Salvito D, Selck H, Ying G-G, Van den Brink PJ (2020) Benthic invertebrate and microbial biodiversity in sub-tropical urban rivers: correlations with environmental variables and emerging chemicals. Sci Total Environ 709:136281. https://doi.org/10.1016/j.scitotenv.2019.136281
Pressler BM, Vaden SL, Lane IF, Cowgill LD, Dye JA (2003) Candida spp. urinary tract infections in 13 dogs and seven cats: predisposing factors, treatment, and outcome. J Am Anim Hosp Assoc 39:263–270. https://doi.org/10.5326/0390263
Reagan KL, Dear JD, Kass PH, Sykes JE (2019) Risk factors for Candida urinary tract infections in dogs and cats. J Vet Intern Med 33:648–653. https://doi.org/10.1111/jvim.15444
Elmberg J, Berg C, Lerner H, Waldenström J, Hessel R (2017) Potential disease transmission from wild geese and swans to livestock, poultry and humans: a review of the scientific literature from a one health perspective. Infect Ecol Epidemiol 7:1300450. https://doi.org/10.1080/20008686.2017.1300450
Hillier A, Alcorn JR, Cole LK, Kowalski JJ (2006) Pyoderma caused by Pseudomonas aeruginosa infection in dogs: 20 cases. Vet Dermatol 17:432–439. https://doi.org/10.1111/j.1365-3164.2006.00550.x
Siqueira AK, Ribeiro MG, Leite D d S, Tiba MR, de Moura C, Lopes MD, Prestes NC, Salerno T, Silva AV d (2009) Virulence factors in Escherichia coli strains isolated from urinary tract infection and pyometra cases and from feces of healthy dogs. Res Vet Sci 86:206–210. https://doi.org/10.1016/j.rvsc.2008.07.018
Zhou L, Kassa H, Tischler ML, Xiao L (2004) Host-adapted Cryptosporidium spp. in Canada Geese (Branta canadensis). Appl Environ Microbiol 70:4211–4215. https://doi.org/10.1128/AEM.70.7.4211-4215.2004
Sales-Ortells H, Medema G (2014) Screening-level microbial risk assessment of urban water locations: a tool for prioritization. Environ Sci Technol 48:9780–9789. https://doi.org/10.1021/es5020407
Acknowledgments
The authors would like to thank to the subdirector of the Instituto Distrital de Recreación y Deporte (IDRD), Javier Suarez, for giving us the authorization to collect the water samples in the water bodies of the metropolitan and linear parks of Bogotá. The authors would also like to thank Milena Camargo for her support in sample transportation to the Laboratory of Microbiology at Universidad del Rosario. Finally, the authors thank Enago editing service for editing a draft of this manuscript that was supported by the Dirección de Investigación e Innovación from Universidad del Rosario.
Funding
This work was funded by DIRECCIÓN DE INVESTIGACIÓN E INNOVACIÓN from Universidad del Rosario.
Author information
Authors and Affiliations
Contributions
J.D.R and L.C.S designed the experimental design of the present study. J.J., D.M.M, D.M., and L.V collected and processed the samples in the laboratory. M.M and L.V performed the bioinformatic and descriptive analyzes. M.M and J.D.R revised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Information
Additional file 1
Description: Environmental parameters of the five sampled water bodies. (CSV 244 bytes)
Additional file 2
Coliform cultures belonging to the four URLs and the stream of the study. Red colonies in the Nutrient-TCC agar of (A) Los Novios, (B) Simon Bolivar, (C) Timiza, (D) El Tunal, and (E) El Virrey confirm the presence of aerobic coliforms. (F) El Virrey stream was the only water body that exhibited bacterial growth in the MacConkey agar. Each cross in the agar surface represents a size of 4 mm. (PDF 1857 kb)
Additional file 3
Heatmap of the relative abundance of the bacterial genera classified as “not identified” and underwent a posterior classification with BLASTn. (PDF 553 kb)
Additional file 4
Representation of relative abundances of (A) fungi genera with a relative abundance higher than 1%, within the five water bodies. (B) Representation of the relative abundance of Candida species found within the water bodies. (PDF 264 kb)
Additional file 5
Photographs taken in the metropolitan park Los Novios where (A) citizens take rides in water bicycles within Los Novios URL and where (B) birds such as ducks and geese are observed inhabiting the URL. (PDF 970 kb)
Additional file 6
Photographs taken in the metropolitan park Simon Bolivar where (A) citizen take rides in boats and (B) in water bicycles within the URL where some waterborne pathogens are present. (PDF 831 kb)
Additional file 7
Photographs taken in the metropolitan park Timiza where (A) birds such as geese and ducks can be seen swimming in the URL. (B) photograph of pets in close contact with the URL. (PDF 954 kb)
Additional file 8
Photographs taken in the metropolitan park El Tunal where (A) pets enter the water of the URL, which shows waterborne pathogens. (B) The fountain at this URL might facilitate the airborne transmission of some pathogens by aerosols or microdroplets. (PDF 750 kb)
Additional file 9
Photographs taken in the linear park El Virrey where (A) El Virrey stream can be visualized as a shallow water body with contamination by sewage water and also (B) the stream contamination by solid waste. (PDF 1014 kb)
Rights and permissions
About this article
Cite this article
Vega, L., Jaimes, J., Morales, D. et al. Microbial Communities’ Characterization in Urban Recreational Surface Waters Using Next Generation Sequencing. Microb Ecol 81, 847–863 (2021). https://doi.org/10.1007/s00248-020-01649-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-020-01649-9