Abstract
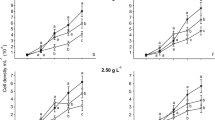
The effect of three different nutritional conditions during the initial 12 h of interaction between the microalgae Chlorella sorokiniana UTEX 2714 and the plant growth–promoting bacterium Azospirillum brasilense Cd on formation of synthetic mutualism was assessed by changes in population growth, production of signal molecules tryptophan and indole-3-acetic acid, starch accumulation, and patterns of cell aggregation. When the interaction was supported by a nutrient-rich medium, production of both signal molecules was detected, but not when this interaction began with nitrogen-free (N-free) or carbon-free (C-free) media. Overall, populations of bacteria and microalgae were larger when co-immobilized. However, the highest starch production was measured in C. sorokiniana immobilized alone and growing continuously in a C-free mineral medium. In this interaction, the initial nutritional condition influenced the time at which the highest accumulation of starch occurred in Chlorella, where the N-free medium induced faster starch production and the richer medium delayed its accumulation. Formation of aggregates made of microalgae and bacteria occurred in all nutritional conditions, with maximum at 83 h in mineral medium, and coincided with declining starch content. This study demonstrates that synthetic mutualism between C. sorokiniana and A. brasilense can be modulated by the initial nutritional condition, mainly by the presence or absence of nitrogen and carbon in the medium in which they are interacting.







Similar content being viewed by others
References
Sapp M, Schwaderer AS, Wiltshire KH, Hoppe H-G, Gerdts G, Wichels A (2007) Species-specific bacterial communities in the phycosphere of microalgae? Microb Ecol 53:683–699. https://doi.org/10.1007/s00248-006-9162-5
Kouzuma A, Watanabe K (2015) Exploring the potential of algae/bacteria interactions. Curr Opin Biotech 33:125–129. https://doi.org/10.1016/j.copbio.2015.02.007
Harcombe WR, Riehl WJ, Dukovski I, Granger BR, Betts A, Lang AH, Bonilla G, Kar A, Leiby N, Mehta P, Marx CJ, Segrè D (2014) Metabolic resource allocation in individual microbes determines ecosystem interactions and spatial dynamics. Cell Rep 7:1104–1115. https://doi.org/10.1016/j.celrep.2014.03.070
de-Bashan LE, Mayali X, Bebout BM, Weber PK, Detweiler AM, Hernandez J-P, Prufert-Bebout L, Bashan Y (2016) Establishment of stable synthetic mutualism without co-evolution between microalgae and bacteria demonstrated by mutual transfer of metabolites (NanoSIMS isotopic imaging) and persistent physical association (fluorescent in situ hybridization). Algal Res 15:179–186. https://doi.org/10.1016/j.algal.2016.02.019
Dolinšek J, Goldschmidt F, Johnson DR (2016) Synthetic microbial ecology and the dynamic interplay between microbial genotypes. FEMS Microbiol Rev 40:961–979. https://doi.org/10.1093/femsre/fuw024
Goers L, Freemont P, Polizzi KM (2014) Co-culture systems and technologies: taking synthetic biology to the next level. J Roy Soc Interface 11:20140065. https://doi.org/10.1098/rsif.2014.0065
Ramanan R, Kim B-H, Cho D-H, Oh H-M, Kim H-S (2016) Algae-bacteria interactions: evolution, ecology and emerging applications. Biotechnol Lett 34:14–29. https://doi.org/10.1016/j.biotechadv.2015.12.003
Kazamia E, Czesnick H, Van Nguyen TT, Croft MT, Sherwood E, Sasso S et al (2012) Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ Microbiol 14:1466–1476. https://doi.org/10.1111/j.1462-2920.2012.02733.x
Xie B, Bishop S, Stessman D, Wright D, Spalding MH, Halverson LJ (2013) Chlamydomonas reinhardtii thermal tolerance enhancement mediated by a mutualistic interaction with vitamin B12-producing bacteria. ISME J 7:1544–1555. https://doi.org/10.1038/ismej.2013.43
Zhou K, Qiao K, Edgar S, Stephanopoulos G (2015) Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol 33:377–385. https://doi.org/10.1038/nbt.3095
Gonzalez LE, Bashan Y (2000) Growth promotion of the microalgae Chlorella vulgaris when coimmobilized and cocultured in alginated beads with the plant growth-promoting bacteria Azospirillum brasilense. Appl Environ Microbiol 66:1537–1541. https://doi.org/10.1128/AEM.66.4.1527-1531.20
de-Bashan LE, Bashan Y (2008) Joint immobilization of plant growth-promoting bacteria and green microalgae in alginate beads as an experimental model for studying plant-bacterium interactions. Appl Environ Microb 74:6797–6802. https://doi.org/10.1128/AEM.00518-08
de-Bashan LE, Schmid M, Rothballer M, Hartmann A, Bashan Y (2011) Cell-cell interaction in the eukaryote–prokaryote model of the microalgae Chlorella vulgaris and the bacterium Azospirillum brasilense immobilized in polymer beads. J Phycol 47:1350–1359. https://doi.org/10.1111/j.1529-8817.2011.01062.x
de-Bashan LE, Hernandez J-P, Bashan Y (2015) Interaction of Azospirillum spp. with microalgae; a basic eukaryotic–prokaryotic model and its biotechnological applications. In: Cassán FD, Okon Y, Creus CM (Eds) Handbook for Azospirillum. Technical issued and protocols, Springer International Publishing, Switzerland, pp 367–388
Lebsky VK, Gonzalez-Bashan LE, Bashan Y (2001) Ultrastructure of coinmmobilization of the microalgae Chlorella vulgaris with the plant growth-promoting bacterium Azospirillum brasilense and with its natural associative bacterium Phyllobacterium myrsinacearum in alginate beads. Can J Microbiol 47:1–8. https://doi.org/10.1139/w00-115
de-Bashan LE, Bashan Y, Moreno M, Lebsky VK, Bustillos JJ (2002) Increased pigment and lipid content, lipid variety, and cell and population size of the microalgae Chlorella spp. when co-immobilized in alginate beads with the microalgae-growth-promoting bacterium Azospirillum brasilense. Can J Microbiol 48:514–521. https://doi.org/10.1139/w02-051
de-Bashan LE, Magallon P, Antoun H, Bashan Y (2008) Role of glutamate dehydrogenase and glutamine synthetase in Chlorella vulgaris during assimilation of ammonium when jointly immobilized with the microalgae-growth-promoting bacterium Azospirillum brasilense. J Phycol 44:1188–1196. https://doi.org/10.1111/j.1529-8817.2008.00572.x
Choix FJ, de-Bashan LE, Bashan Y (2012) Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: I. autotrophic conditions. Enzyme Microb Tech 51:294–299. https://doi.org/10.1016/j.enzmictec.2012.07.013
Leyva LA, Bashan Y, Mendoza A, de-Bashan LE (2014) Accumulation of fatty acids in Chlorella vulgaris under heterotrophic conditions in relation to activity of acetyl-CoA carboxylase, temperature, and co-immobilization with Azospirillum brasilense. Naturewissenschaften 101:819–830. https://doi.org/10.1007/s00114-014-1223-x
Palacios OA, Bashan Y, Schmid M, Hartmann A, de-Bashan LE (2016a) Enhancement of thiamine release during synthetic mutualism between Chlorella sorokiniana and Azospirillum brasilense growing under stress conditions. J Appl Phycol 28:1521–1531. https://doi.org/10.1007/s10811-015-0697-z
Palacios OA, Choix FC, Bashan Y, de-Bashan LE (2016b) Influence of tryptophan and indole-3-acetic acid on starch accumulation in synthetic mutualistic Chlorella sorokiniana – Azospirillum brasilense system under heterotrophic conditions. Res Microbiol 167:367–379. https://doi.org/10.1016/j.resmic.2016.02.005
de-Bashan LE, Moreno M, Hernandez JP, Bashan Y (2002) Removal of ammonium and phosphorous ions from synthetic wastewater by the microalgae Chlorella vulgaris coimmobilized in alginate beads with the microalgae growth-promoting bacterium Azospirillum brasilense. Water Res 36:2941–2948. https://doi.org/10.1016/S0043-1354(01)00522-X
de-Bashan LE, Hernandez JP, Morey T, Bashan Y (2004) Microalgae growth-promoting bacteria as “helpers” for microalgae: a novel approach for removing ammonium and phosphorous for municipal wastewater. Water Res 38:466–474. https://doi.org/10.1016/j.watres.2003.09.022
Choix FJ, López-Cisneros GC, Méndez-Acosta HO (2018) Azospirillum brasilense increase CO2 fixation on microalgae Scenedesmus obliquus, Chlorella vulgaris, and Chlamydomonas reinhardtii cultured on high CO2 concentrations. Microb Ecol.https://doi.org/10.1007/s00248-017-1139-z
Bashan Y (1986) Alginate beads as synthetic inoculant carriers for the slow release of bacteria that affect plant growth. Appl Environ Microb 51:1089–1098
Bashan Y, Lopez BR, Volker AR, Amavizca E, de-Bashan LE (2016) Chlorella sorokiniana (formerly C. vulgaris) UTEX 2714, a non-thermotolerant microalgae useful for biotechnological applications and as a reference strain. J Appl Phycol 28:113–121. https://doi.org/10.1007/s10811-015-0571-z
Gonzalez LE, Cañizares RO, Baena S (1997) Efficiency of ammonia and phosphorous removal from a Colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresour Technol 60:259–262. https://doi.org/10.1016/S0960-8524(97)00029-1
Bashan Y, Trejo A, de-Bashan LE (2011) Development of two culture media for mass cultivation of Azospirillum spp. and for production of inoculants to enhance plant growth. Biol Fertil Soils 47:963–969. https://doi.org/10.1007/s00374-011-0555-3
de-Bashan LE, Bashan Y (2010) Immobilized microalgae for removing pollutants: review of practical aspects. Bioresour Technol 101:1611–1627. https://doi.org/10.1016/j.biortech.2009.09.043
Bashan Y, Holguin G, Lifshitz R (1993) Isolation and characterization of plant growth-promoting rhizobacteria. In: Glick BR, Thompson JE (eds) Methods in plant molecular biology and biotechnology. CRC Press, Boca Raton, pp 331–345
Chrzanowski TH, Crotty RD, Hubbard JG, Welch RP (1984) Applicability of fluorescein diacetate method of detecting active bacteria in freshwater. Microb Ecol 10:179–185. https://doi.org/10.1007/BF02011424
Zakharova EA, Shcherbakov AA, Brudnik VV, Skipko NG, Bulkhin NS, Ignatov VV (1999) Biosynthesis of indole-3-acetic acid in Azospirillum brasilense. Insights from quantum chemistry. Eur J Biochem 259:572–576. https://doi.org/10.1046/j.1432-1327.1999.00033.x
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microb 56:1919–1925
Daims H, Brühl A, Amann R, Schleifer KH, Wagner M (1999) The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22:434–444. https://doi.org/10.1016/S0723-2020(99)80053-8
Stoffels M, Castellanos T, Hartmann A (2001) Design and application of new 16S rRNA-targeted oligonucleotide probes for the Azospirillum-Skermanella-Rhodocista-cluster. Syst Appl Microbiol 24:83–97
Bronstein JL (1994) Conditional outcomes in mutualistic interactions. Trends Ecol Evol 9:214–217. https://doi.org/10.1016/0169-5347(94)90246-1
Krediet CJ, Ritchie KB, Paul VJ, Teplitski M (2013) Coral-associated micro-organisms and their roles in promoting coral health and thwarting diseases. P Roy Soc B 280:20122328. https://doi.org/10.1098/rspb.2012.2328
de Mazancourt C, Loreau M, Dieckmann U (2005) Understanding mutualism when there is adaptation to the partner. J Ecol 93:305–314. https://doi.org/10.1111/j.0022-0477.2004.00952.x
Wäckers FL, Alberola JS, Garcia-Marí F, Pekas A (2017) Attract and distract: manipulation of a food-mediated protective mutualism enhances natural pest control. Agric Ecosyst Environ 246:168–174. https://doi.org/10.1016/j.agee.2017.05.037
Zuroff TR, Xiques SB, Curtis WR (2013) Consortia-mediated bioprocessing of cellulose to ethanol with a symbiotic Clostridium phytofermentans/yeast co-culture. Biotechnol Biofuels 6:59. https://doi.org/10.1186/1754-6834-6-59
Palacios OA, Gomez-Anduro G, Bashan Y, de-Bashan LE (2016c) Tryptophan, thiamine and indole-3-acetic acid exchange between Chlorella sorokiniana and the plant growth-promoting bacterium Azospirillum brasilense. FEMS Microbiol Ecol 92:fiw077. https://doi.org/10.1093/femsec/fiw077
Tarrand JJ, Krieg NR, Döbereiner J (1978) A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerink) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol 24:967–980. https://doi.org/10.1139/m78-160
Bashan Y, Levanony H, Klein E (1986) Evidence for a weak active external adsorption of Azospirillum brasilense Cd to wheat roots. J Gen Microbiol 132:3069–3073. https://doi.org/10.1099/00221287-132-11-3069
Pereg L, de-Bashan LE, Bashan Y (2016) Assessment of affinity and specificity of Azospirillum for plants. Plant Soil 399:389–414. https://doi.org/10.1007/s11104-015-2778-9
Michiels KW, Croes CL, Vanderleyden J (1991) Two different modes of attachment of Azospirillum brasilense Sp7 to wheat roots. J Gen Microbiol 137:2241–2246. https://doi.org/10.1099/00221287-137-9-2241
Schnurr PJ, Allen DG (2015) Factors affecting algae biofilm growth and lipid production: a review. Renew Sust Energ Rev 52:418–429. https://doi.org/10.1016/J.rser.2015.07.090
Shen Y, Zhang H, Xu X, Lin X (2015) Biofilm formation and lipid accumulation of attached culture of Botryococcus braunii. Bioprocess Biosyst Eng 38:481–488. https://doi.org/10.1007/s00449-014-1287-1
Bashan Y, de-Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant-growth? – a critical assessment. Adv Agron 108:77–136. https://doi.org/10.1016/S0065-2113(10)08002-8
Zhu S, Huang W, Xu J, Wang Z, Xu J, Yuan Z (2014) Metabolic changes of starch and lipid triggered by nitrogen starvation in the microalga Chlorella zofingiensis. Bioresour Technol 152:292–298. https://doi.org/10.1016/j.biortech.2013.10.092
Park J-J, Wang H, Gargouri M, Deshpande RR, Skepper JN, Holguin O et al (2015) The response of Chlamydomonas reinhardtii to nitrogen deprivation: a systems biology analysis. Plant Biol 81:611–624
Zhang X, Reed JL (2014) Adaptive evolution of synthetic cooperating communities improves growth performance. PLoS One 9:e108297. https://doi.org/10.1111/tpj.12747
Klitgord N, Segrè D (2010) Environments that induce synthetic microbial ecosystems. PLoS Comput Biol 6:e1001002. https://doi.org/10.1371/journal.pcbi.1001002
Morris BEL, Henneberger R, Huber H, Moissl-Eichinger C (2013) Microbial syntrophy: interaction for the common good. FEMS Microbiol Rev 37:384–406. https://doi.org/10.1111/1574-6976.12019
Choix FJ, Bashan Y, Mendoza A, de-Bashan LE (2014) Enhanced activity of ADP glucose pyrophosphorylase and formation of starch induced by Azospirillum brasilense in Chlorella vulgaris. J Biotechnol 177:22–34. https://doi.org/10.1016/j.biotec.2014.02.014
Wu H, Miao X (2014) Biodiesel quality and biochemical changes of microalgae Chlorella pyrenoidosa and Scenedesmus obliquus in response to nitrate levels. Bioresour Technol 170:421–427. https://doi.org/10.1016/j.biortech.2014.08.017
González-Fernández C, Ballesteros M (2012) Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol Adv 30:1655–1661. https://doi.org/10.1016/j.biotechadv.2012.07.003
Acknowledgments
At CIBNOR, Mexico, we thank Manuel Moreno, Francisco Hernandez, and Patricia Hinojosa for technical assistance.
Funding
This work was financially supported by Consejo Nacional de Ciencia y Tecnología of Mexico (CONACYT Basic Science-2015, contract 251102 and CONACYT Basic Science-2017, contract 284562) and the actual drafting of the paper was supported by The Bashan Foundation, USA. This is contribution 2018-027 of the Bashan Institute of Science, USA.
Author information
Authors and Affiliations
Contributions
OAP and BRL designed and executed the study and wrote the initial draft. YB critically revised the article for intellectual content including the final version. LEdeB designed and managed the project and critically revised the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
This study is dedicated in memory of Dr. Michael Schmid (Big Mike) (1968–2016) of Helmholtz Zentrum Münich, Neuherberg, Germany
Prof. Yoav Bashan passed away during the revision of this manuscript
The text was edited by a professional editing services company (Scribendi, Canada).
Electronic Supplementary Material
Table S1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Palacios, O.A., Lopez, B.R., Bashan, Y. et al. Early Changes in Nutritional Conditions Affect Formation of Synthetic Mutualism Between Chlorella sorokiniana and the Bacterium Azospirillum brasilense. Microb Ecol 77, 980–992 (2019). https://doi.org/10.1007/s00248-018-1282-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-018-1282-1