Abstract
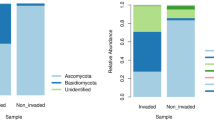
The death of trees is an ecological process that promotes regeneration, organic matter recycling, and the structure of communities. However, diverse biotic and abiotic factors can disturb this process. Dendroctonus bark beetles (Curculionidae: Scolytinae) are natural inhabitants of pine forests, some of which produce periodic outbreaks, killing thousands of trees in the process. These insects spend almost their entire life cycle under tree bark, where they reproduce and feed on phloem. Tunneling and feeding of the beetles result in the death of the tree and an alteration of the resident microbiota as well as the introduction of microbes that the beetles vector. To understand how microbial communities in subcortical tissues of pines change after they are colonized by the bark beetle Dendroctonus rhizophagus, we compare both the bacterial and fungal community structures in two colonization stages of Pinus arizonica (Arizona pine) employing Illumina MiSeq. Our findings showed significant differences in diversity and the dominance of bacterial community in the two colonization stages with Shannon (P = 0.004) and Simpson (P = 0.0006) indices, respectively, but not in species richness with Chao1 (P = 0.19). In contrast, fungal communities in both stages showed significant differences in species richness with Chao1 (P = 0.0003) and a diversity with Shannon index (P = 0.038), but not in the dominance with the Simpson index (P = 0.12). The β-diversity also showed significant changes in the structure of bacterial and fungal communities along the colonization stages, maintaining the dominant members in both cases. Our results suggest that microbial communities present in the Arizona pine at the tree early colonization stage by bark beetle change predictably over time.




Similar content being viewed by others
References
Franklin JF, Shugart HH, Harmon ME (1987) Tree death as an ecological process. BioScience 37:550–556
Castello JD, Leopold DJ, Smallidge PJ (1995) Pathogens, patterns, and processes in forest ecosystems. BioScience 45:16–24
Anderegg WRL, Kane JM, Anderegg LDL (2013) Consequences of widespread tree mortality triggered by drought and temperature stress. Nat Clim Chang 3:30–36
Wood SL (1982) The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Nat Mem 6:1–1356. https://doi.org/10.1038/NCLIMATE1635
Raffa KF, Aukema BH, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH (2008) Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. BioScience 58:501–517. https://doi.org/10.1641/B580607
Blomquist GJ, Figueroa-Teran R, Aw M, Song M, Gorzalski A, Abbott NL, Chang E, Tittiger C (2010) Pheromone production in bark beetles. Insect Biochem Mol Biol 40:699–712. https://doi.org/10.1016/j.ibmb.2010.07.013
Fettig CJ, Klepzig KD, Billings RF, Munson AS, Nebeker TE, Negrón JF, Nowak JT (2007) The effectiveness of vegetation management practices for prevention and control of bark beetle infestations in coniferous forests of the western and southern United States. For Ecol Manag 238:24–53. https://doi.org/10.1016/J.FORECO.2006.10.011
Parmeter JR, Slaughter GW, Chen MM, Wood DL, Stubb HA (1989) Single and mixed inoculations of ponderosa pine with fungal associates of Dendroctonus spp. Phytopathology 79:768–772. https://doi.org/10.1094/Phyto-79-768
Paine TD, Raffa KF, Harrington TC (1997) Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu Rev Entomol 42:179–206. https://doi.org/10.1146/annurev.ento.42.1.179
Lewinsohn D, Lewinsohn E, Bertagnolli CL, Patridge AD (1994) Blue-stain fungi and their transport structures on the Douglas fir beetle. Can J For Res 24:2275–2283. https://doi.org/10.1139/x94-292
Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167:353–376. https://doi.org/10.1111/j.1469-8137.2005.01436.x
Keeling CI, Bohlmann J (2006) Diterpene resin acids in conifers. Phytochemistry 67:2415–2423. https://doi.org/10.1016/J.PHYTOCHEM.2006.08.019
Redford AJ, Bowers RM, Knight R, Linhart Y, Fierer N (2010) The ecology of the phyllosphere: geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ Microbiol 12:2885–2893. https://doi.org/10.1111/j.1462-2920.2010.02258.x
Turner TR, James EK, Poole PS (2013) The plant microbiome. Genome Biol 14:209. https://doi.org/10.1186/gb-2013-14-6-209
Strid Y, Schroeder M, Lindahl B, Ihrmark K, Stenlid J (2014) Bark beetles have a decisive impact on fungal communities in Norway spruce stem sections. Fungal Ecol 7:47–58. https://doi.org/10.1016/j.funeco.2013.09.003
Rivera FN, González E, Gomez Z, López N, Hernández-Rodríguez C, Berkov A, Zúñiga G (2009) Gut-associated yeast in bark beetles of the genus Dendroctonus Erichson (Coleoptera: Curculionidae: Scolytinae). Biol J Linn Soc 98:325–342. https://doi.org/10.1111/j.1095-8312.2009.01289.x
Durand AA, Bergeron A, Constant P, Buffet JP, Déziel E, Guertin C (2015) Surveying the endomicrobiome and ectomicrobiome of bark beetles: the case of Dendroctonus simplex. Sci Rep 5:17190. https://doi.org/10.1038/srep17190
Briones-Roblero CI, Hernández-García JA, Gonzalez-Escobedo R, Soto-Robles LV, Rivera-Orduña FN, Zúñiga G (2017) Structure and dynamics of the gut bacterial microbiota of the bark beetle, Dendroctonus rhizophagus (Curculionidae: Scolytinae) across their life stages. PLoS One 12:e0175470. https://doi.org/10.1371/journal.pone.0175470
Hernández-García JA, Briones-Roblero CI, Rivera-Orduña FN, Zúñiga G (2017) Revealing the gut bacteriome of Dendroctonus bark beetles (Curculionidae: Scolytinae): diversity, core members and co-evolutionary patterns. Sci Rep 7(13864):13864. https://doi.org/10.1038/s41598-017-14031-6
Bleiker KP, Potter SE, Lauzon CR, Six DL (2009) Transport of fungal symbionts by mountain pine beetles. Can Entomol 141:503–514. https://doi.org/10.4039/n09-034
Bracewell RR, Six DL (2014) Broadscale specificity in a bark beetle-fungal symbiosis: a spatio-temporal analysis of the mycangial fungi of the western pine beetle. Microb Ecol 68:859–870. https://doi.org/10.1007/s00248-014-0449-7
Klepzig KD, Hofstetter RW (2011) From attack to emergence: interactions between the southern pine beetle, mites, microbes, and trees. In: Coulson RN, Klepzig KD (eds) Southern pine beetle II. General Technical Report SRS-140. Department of Agriculture Forest Service, Southern Research Station, Asheville, pp 141–152
Hofstetter RW, Dinkins-Bookwalter J, Davis TS, Klepzig KD (2015) Symbiotic associations of bark beetles. In: Vega FE, Hofstetter RW (eds) Bark Beetles. Elsevier, Amsterdam, pp 209–245. https://doi.org/10.1016/B978-0-12-417156-5.00006-X
Carrell AA, Frank AC (2014) Pinus flexilis and Picea engelmannii share a simple and consistent needle endophyte microbiota with a potential role in nitrogen fixation. Front Microbiol 5:333. https://doi.org/10.3389/fmicb.2014.00333
Carrell AA, Frank AC (2015) Bacterial endophyte communities in the foliage of coast redwood and giant sequoia. Front Microbiol 6:e01008. https://doi.org/10.3389/fmicb.2015.01008
Carrell AA, Carper DL, Frank AC (2016) Subalpine conifers in different geographical locations host highly similar foliar bacterial endophyte communities. FEMS Microbiol Ecol 92:fiw124. https://doi.org/10.1093/femsec/fiw124
Rúa MA, Wilson EC, Steele S, Munters AR, Hoeksema JD, Frank AC (2016) Associations between ectomycorrhizal fungi and bacterial needle endophytes in Pinus radiata: implications for biotic selection of microbial communities. Front Microbiol 7:399. https://doi.org/10.3389/fmicb.2016.00399
Gonzalez-Escobedo R, Briones-Roblero CI, Pineda-Mendoza RM, Rivera-Orduña FN, Zúñiga G (2018) Bacteriome from Pinus arizonica and P. durangensis: diversity, comparison of assemblages, and overlapping degree with the gut bacterial community of a bark beetle that kills pines. Front Microbiol 9:77. https://doi.org/10.3389/fmicb.2018.00077
Roth M, Hussain A, Cale JA, Erbilgin N (2018) Successful colonization of lodgepole pine trees by mountain pine beetle increased monoterpene production and exhausted carbohydrate reserves. J Chem Ecol 44:209–214. https://doi.org/10.1007/s10886-017-0922-0
Mendoza MG, Salinas-Moreno Y, Olivo-Martínez A, Zúñiga G (2011) Factors influencing the geographical distribution of Dendroctonus rhizophagus (Coleoptera: Curculionidae: Scolytinae) in the Sierra Madre Occidental, Mexico. Environ Entomol 40:549–559. https://doi.org/10.1603/EN10059
Herlemann DP, Labrenz M, Jürgens K, Bertilsson S, Waniek JJ, Andersson AF (2011) Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J 5:1571–1579. https://doi.org/10.1038/ismej.2011.41
Bokulich NA, Mills DA (2013) Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl Environ Microbiol 79:2519–2526. https://doi.org/10.1128/AEM.03870-12
Magoc T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. https://doi.org/10.7717/peerj.2584
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. https://doi.org/10.1128/AEM.00062-07
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Dueñas M, Grebenc T, Griffith GW, Hartmann M, Kirk PM, Kohout P, Larsson E, Lindahl BD, Lücking R, Martín MP, Matheny PB, Nguyen NH, Niskanen T, Oja J, Peay KG, Peintner U, Peterson M, Põldmaa K, Saag L, Saar I, Schüßler A, Scott JA, Senés C, Smith ME, Suija A, Taylor DL, Telleria MT, Weiss M, Larsson KH (2013) Towards a unified paradigm for sequence-based identification of Fungi. Mol Ecol 22:5271–5277. https://doi.org/10.1111/mec.12481
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol Microbiol 67:1613–1617. https://doi.org/10.1099/ijsem.0.001755
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Oliveros JC (2007) VENNY. An interactive tool for comparing lists with Venn diagrams. http://bioinfogp.cnb.csic.es/tools/venny/index.html Accessed 26 March 2018
Chao A (1984) Non-parametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Shannon CA (1948) Mathematical theory of communication. Bell Syst Tech J 27:379–423
Simpson EH (1949) Measurement of diversity. Nature 163:688–688
Good IJ (1953) The population frequencies of species and the estimation of population parameters. Biometrika 40:237–264
Rohlf FJ (1997) NTSYSpc Numerical taxonomy and multivariate analysis system version 2.0 user guide. Applied Biostatistics Inc., East Setauket
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Zhang HB, Yang MX, Tu R (2008) Unexpectedly high bacterial diversity in decaying wood of a conifer as revealed by a molecular method. Int Biodeterior Biodegrad 62:471–474 https://doi.org/10.1016/j.ibiod.2008.06.001
Sun H, Terhonen E, Kasanen R, Asiegbu FO (2014) Diversity and community structure of primary wood-inhabiting bacteria in boreal forest. Geomicrobiol J 31:315–324. https://doi.org/10.1080/01490451.2013.827763
Hoppe B, Krger K, Kahl T, Arnstadt T, Buscot F, Bauhus J, Wubet T (2015) A pyrosequencing insight into sprawling bacterial diversity and community dynamics in decaying deadwood logs of Fagus sylvatica and Picea abies. Sci Rep 5:9456. https://doi.org/10.1038/srep09456
Rinta-Kanto J, Sinkko H, Rajala T et al (2016) Natural decay process affects the abundance and community structure of bacteria and Archaea in Picea abies logs. FEMS Microbiol Ecol 92:403–410. https://doi.org/10.1093/femsec/fiw087
de Boer W, van der Wal A (2008) Interactions between saprotrophic basidiomycetes and bacteria. In: Boddy L, Frankland JC, van West P (eds) Ecology of saprotrophic Basidiomycetes. Elsevier, Amsterdam, pp 143–153
Valášková V, de Boer W, Gunnewiek PJK, Pospíšek M, Baldrian P (2009) Phylogenetic composition and properties of bacteria coexisting with the fungus Hypholoma fasciculare in decaying wood. ISME J 3:1218–1221. https://doi.org/10.1038/ismej.2009.64
Bomberg M, Timonen S (2007) Distribution of Cren- and Euryarchaeota in scots pine mycorrhizospheres and boreal forest humus. Microb Ecol 54:406–416. https://doi.org/10.1007/s00248-007-9232-3
Bomberg M, Timonen S (2009) Effect of tree species and mycorrhizal colonization on the archaeal population of boreal forest rhizospheres. Appl Environ Microbiol 75:308–315. https://doi.org/10.1128/AEM.01739-08
Bates ST, Berg-Lyons DB, Caporosa JG, Walters WA, Knight R, Fierer N (2011) Examining the global distribution of dominant archaeal populations in soils. ISME J 5:908–917. https://doi.org/10.1038/ismej.2010.171
Angel R, Claus P, Conrad R (2012) Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. ISME J 6:847–862. https://doi.org/10.1038/ismej.2011
Parfitt D, Hunt J, Dockrell D, Rogers HJ, Boddy L (2010) Do all trees carry the seeds of their own destruction? PCR reveals numerous wood decay fungi latently present in sapwood of a wide range of angiosperm trees. Fungal Ecol 3:338–346. https://doi.org/10.1016/j.funeco.2010.02.001
Rajala T, Peltoniemi M, Pennanen T, Mäkipää R (2012) Fungal community dynamics in relation to substrate quality of decaying Norway spruce (Picea abies [L.] Karst.) logs in boreal forests. FEMS Microbiol Ecol 81:494–505. https://doi.org/10.1111/j.1574-6941.2012.01376.x
Siitonen J (2001) Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecol Bull 49:11–41. https://doi.org/10.2307/20113262
Lundell TK, Mäkelä MR, de Vries RP, Hildén KS (2014) Chapter eleven - genomics, lifestyles and future prospects of wood-decay and litter-decomposing Basidiomycota. In: Martin FM (ed) Advances in botanical research. Elsevier Academic Press, London, pp 329–370. https://doi.org/10.1016/B978-0-12-397940-7.00011-2
Riley R, Salamov AA, Brown DW, Nagy LG, Floudas D, Held BW, Levasseur A, Lombard V, Morin E, Otillar R, Lindquist EA, Sun H, LaButti KM, Schmutz J, Jabbour D, Luo H, Baker SE, Pisabarro AG, Walton JD, Blanchette RA, Henrissat B, Martin F, Cullen D, Hibbett DS, Grigoriev IV (2014) Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc Natl Acad Sci U S A 111:9923–9928 https://doi.org/10.1073/pnas.1400592111
Johnston SR, Boddy L, Weightman AJ (2016) Bacteria in decomposing wood and their interactions with wood-decay fungi. FEMS Microbiol Ecol 92:fiw179. https://doi.org/10.1093/femsec/fiw179
Adams AS, Aylward FO, Adams SM, Erbilgin N, Aukema BH, Currie CR, Suen G, Raffa KF (2013) Mountain pine beetles colonizing historical and naïve host trees are associated with a bacterial community highly enriched in genes contributing to terpene metabolism. Appl Environ Microbiol 79:3468–3475. https://doi.org/10.1128/AEM.00068-13
Cheng CH, Xu LT, Xu DD, Lou QZ, Lu M, Sun JH (2016) Does cryptic microbiota mitigate pine resistance to an invasive beetle–fungus complex? Implications for invasion potential. Sci Rep 6:33110. https://doi.org/10.1038/srep33110
Wielkopolan B, Obrępalska-Stęplowska A (2016) Three-way interaction among plants, bacteria, and coleopteran insects. Planta 244:313–332. https://doi.org/10.1007/s00425-016-2543-1
Morales-Jiménez J, Zúñiga G, Ramírez-Saad HC, Hernández-Rodríguez C (2012) Gut-associated bacteria throughout the life cycle of the bark beetle Dendroctonus rhizophagus Thomas and Bright (Curculionidae: Scolytinae) and their cellulolytic activities. Microb Ecol 64:268–278. https://doi.org/10.1007/s00248-011-9999-0
Briones-Roblero CI, Rodríguez-Díaz R, Santiago-Cruz JA, Zúñiga G, Rivera-Orduña FN (2017) Degradation capacities of bacteria and yeasts isolated from the gut of Dendroctonus rhizophagus (Curculionidae: Scolytinae). Folia Microbiol 62:1–9. https://doi.org/10.1007/s12223-016-0469-4
Giordano L, Gonthier P, Varese GC, Miserere L, Nicolotti G (2009) Mycobiota inhabiting sapwood of healthy and declining scots pine (Pinus sylvestris L.) trees in the Alps. Fungal Divers 38:69–83
Proença DN, Francisco R, Kublik S, Schöler A, Vestergaard G, Schloter M, Morais PV (2017) The microbiome of endophytic, wood colonizing bacteria from pine trees as affected by pine wilt disease. Sci Rep 7:4205. https://doi.org/10.1038/s41598-017-04141-6
Rayner ADM, Boddy L (1988) Fungal decomposition of wood: its biology and ecology. John Willey & Sons, Chichester
Fukasawa Y, Osono T, Takeda H (2009) Microfungus communities of Japanese beech logs at different stages of decay in a cool temperate deciduous forest. Can J For Res 39:1606–1614. https://doi.org/10.1139/X09-080
Hodge A, Fitter AH (2013) Microbial mediation of plant competition and community structure. Funct Ecol 27:865–875. https://doi.org/10.1111/1365-2435.12002
Zhou F, Lou Q, Wang B, Xu L, Cheng C, Lu M, Sun J (2016) Altered carbohydrates allocation by associated bacteria-fungi interactions in a bark beetle-microbe symbiosis. Sci Rep 6:20135. https://doi.org/10.1038/srep20135
Acknowledgments
The authors would like to thank the COPAMEX for allowing the use of its facilities. This work was part of RG-E Ph.D. dissertation. He is a fellow of CONACyT (275347) and “Beca de Estímulo Institucional de Formación de Investigadores” (BEIFI-IPN). We thank the anonymous reviewers for their valuable comments on our manuscript.
Funding
This work was supported by the Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional (SIP 20180686).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Gonzalez-Escobedo, R., Briones-Roblero, C.I., López, M.F. et al. Changes in the Microbial Community of Pinus arizonica Saplings After Being Colonized by the Bark Beetle Dendroctonus rhizophagus (Curculionidae: Scolytinae). Microb Ecol 78, 102–112 (2019). https://doi.org/10.1007/s00248-018-1274-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-018-1274-1