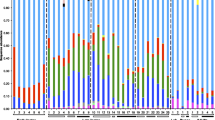
Abstract
Microbiota associated with mosquito vector populations impact several traits of mosquitoes, including survival, reproduction, control, and immunity against pathogens. The influence of seasonal variations and mosquito species on mosquito gut microbiota is poorly understood. We sought to determine whether the mosquito microbiota associated with immature stages of two congeners (Culex coronator and Culex nigripalpus) differ temporally and between the two species. Using high throughput 16S rRNA gene sequence analysis, we characterized bacterial and archaeal communities found in the immature stages of the two Culex mosquito species sampled over three seasons to compare the diversity of bacteria between the two species. Beta diversity analyses of the larval microbiota sequences revealed that the two Culex species differed significantly, both temporally within each species and between the two species. Bacteria in Cx. coronator larvae were dominated by Alphaproteobacteria, mainly associated with Roseoccocus and unidentified species of Rhizobiales, and two unidentified species of Cyanobacteria. In contrast, Cx. nigripalpus was dominated by Thorsellia anophelis (Gammaproteobacteria), Clostridium, an unidentified species of Ruminococcacae (Clostridiales), and additional unidentified species associated with Erysipelotrichaceae (Erysipelotrichales), Bacteroidales, and Mollicutes. Results of our study revealed both seasonal and interspecies differences in bacterial community composition associated with the immature stages of Cx. coronator and Cx. nigripalpus vector populations in Florida. These results have important implications for our understanding of the underlying factors of variations in disease transmission among seasons, susceptibility to various pesticides, and other biotic factors, including the role of the microbiota on the spread of invasive species. In addition, our results suggest close associations of certain bacteria species with each of the two Culex species that will be further targeted for their potential in the development of microbial-based control strategies.






Similar content being viewed by others
References
Coon KL, Vogel KJ, Brown MR, Strand MR (2014) Mosquitoes rely on their gut microbiota for development. Mol. Ecol. 23(11):2727–2739. doi:10.1111/mec.12771
Mitraka E, Stathopoulos S, Siden-Kiamos I, Christophides GK, Louis C (2013) Asaia accelerates larval development of Anopheles gambiae. Pathog Glob Health 107(6):305–311. doi:10.1179/2047773213Y.0000000106
Diaz-Nieto LM, DA C, Perotti MA, Beron CM (2016) Culex pipiens development is greatly influenced by native bacteria and exogenous yeast. PLoS One 11(4):e0153133. doi:10.1371/journal.pone.0153133
Merritt RW, Dadd RH, Walker ED (1992) Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu. Rev. Entomol. 37(1):349–374. doi:10.1146/annurev.en.37.010192.002025
Glaser RL, Meola MA (2010) The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One 5(8):e11977. doi:10.1371/journal.pone.0011977
Finney CA, Kamhawi S, Wasmuth JD (2015) Does the arthropod microbiota impact the establishment of vector-borne diseases in mammalian hosts? PLoS Pathog. 11(4):e1004646. doi:10.1371/journal.ppat.1004646
Zink SD, Van Slyke GA, Palumbo MJ, Kramer LD, Ciota AT (2015) Exposure to West Nile virus increases bacterial diversity and immune gene expression in Culex pipiens. Viruses 7(10):5619–5631. doi:10.3390/v7102886
Dennison NJ, Jupatanakul N, Dimopoulos G (2014) The mosquito microbiota influences vector competence for human pathogens. Curr Opin Insect Sci 3:6–13. doi:10.1016/j.cois.2014.07.004
Brownlie JC, Johnson KN (2009) Symbiont-mediated protection in insect hosts. Trends Microbiol. 17(8):348–354. doi:10.1016/j.tim.2009.05.005
Berticat C, Rousset F, Raymond M, Berthomieu A, Weill M (2002) High Wolbachia density in insecticide-resistant mosquitoes. Proc. Biol. Sci. 269:1413–1416. doi:10.1098/rspb.2002.2022
Duron O, Labbé P, Berticat C, Rousset F, Guillot S, Raymond M, Weill M (2006) High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution 60:303–314. doi:10.1554/05-340.1
Patil CD, Borase HP, Salunke BK, Patil SV (2013) Alteration in Bacillus thuringiensis toxicity by curing gut flora: novel approach for mosquito resistance management. Parasit Res 112(9):3283–3288. doi:10.1007/s00436-013-3507-z
Feldhaar H (2011) Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol Entomol 36(5):533–543. doi:10.1111/j.1365-2311.2011.01318.x
Coon KL, Brown MR, Strand MR (2016) Gut bacteria differentially affect egg production in the anautogenous mosquito Aedes aegypti and facultatively autogenous mosquito Aedes atropalpus (Diptera: Culicidae). Parasit. Vectors 9(1):375. doi:10.1186/s13071-016-1660-9
Pumpuni CB, DeMaio J, Kent M, Davis JR, Beier JC (1996) Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am.J.Trop. Med. Hyg. 54(2):214–218
DeMaio J, Pumpuni CB, Kent M, Beier JC (1996) The midgut bacterial flora of wild Aedes triseriatus, Culex pipiens, and Psorophora columbiae mosquitoes. Am.J.Trop. Med. Hyg. 54(2):219–223
Wang Y, Gilbreath T, Kukutla P, Yan G, Xu J (2011) Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS One 6 (9). doi:10.1371/journal.pone.0024767
Duguma D, Hall MW, Rugman-Jones P, Stouthamer R, Terenius O, Neufeld JD, Walton WE (2015) Developmental succession of the microbiome of Culex mosquitoes. BMC Microbiol. 15:140. doi:10.1186/s12866-015-0475-8
Rani A, Sharma A, Rajagopal R, Adak T, Bhatnagar RK (2009) Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture-independent methods in lab-reared and field-collected Anopheles stephensi—an Asian malarial vector. BMC Microbiol. 9:96. doi:10.1186/1471-2180-9-96
Moll RM, Romoser WS, Modrzakowski MC, Moncayo AC, Lerdthusnee K (2001) Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J. Med. Entomol. 38(1):29–32. doi:10.1603/0022-2585-38.1.29
Chavshin AR, Oshaghi MA, Vatandoost H, Yakhchali B, Zarenejad F, Terenius O (2015) Malpighian tubules are important determinants of Pseudomonas transstadial transmission and longtime persistence in Anopheles stephensi. Parasit. Vectors 8:36. doi:10.1186/s13071-015-0635-6
Gimonneau G, Tchioffo MT, Abate L, Boissiere A, Awono-Ambene PH, Nsango SE, Christen R, Morlais I (2014) Composition of Anopheles coluzzii and Anopheles gambiae microbiota from larval to adult stages. Infect. Genet. Evol. 28:715–724. doi:10.1016/j.meegid.2014.09.029
Wilke AB, Marrelli MT (2015) Paratransgenesis: a promising new strategy for mosquito vector control. Parasit. Vectors 8:342. doi:10.1186/s13071-015-0959-2
Vitek CJ, Richards SL, Mores CN, Day JF, Lord CC (2008) Arbovirus transmission by Culex nigripalpus in Florida, 2005. J. Med. Entomol. 45(3):483–493. doi:10.1603/0022-2585(2008)45[483:ATBCNI]2.0.CO;2
Day JF, Stark LM (2000) Frequency of Saint Louis encephalitis virus in humans from Florida, USA: 1990-1999. J. Med. Entomol. 37(4):626–633. doi:10.1603/0022-2585-37.4.626
Rutledge CR, Day JF, Lord CC, Stark LM, Tabachnick WJ (2003) West Nile virus infection rates in Culex nigripalpus (Diptera: Culicidae) do not reflect transmission rates in Florida. J. Med. Entomol. 40(3):253–258. doi:10.1603/0022-2585-40.3.253
Duguma D, Hall MW, Smartt CT, Neufeld JD (2017) Effects of organic amendments on microbiota associated with the Culex nigripalpus mosquito vector of the Saint Louis encephalitis and West Nile viruses. mSphere 2 (1). doi:10.1128/mSphere.00387-16
Day JF, Curtis GA (1994) When it rains, they soar—and that makes Culex nigripalpus a dangerous mosquito. Am. Entomol. 40(3):162–167. doi:10.1093/ae/40.3.162
O'Meara GF, Cutwa-Francis M, Rey JR (2010) Seasonal variation in the abundance of Culex nigripalpus and Culex quinquefasciatus in wastewater ponds at two Florida dairies. J. Am. Mosq. Control Assoc. 26(2):160–166. doi:10.2987/09-5971.1
Rey JR, O'Meara GF, O'Connell SM, Cutwa-Francis MM (2006) Factors affecting mosquito production from stormwater drains and catch basins in two Florida cities. J Vector Ecol. 31(2):334–343. doi:10.3376/1081-1710(2006)31[334:FAMPFS]2.0.CO;2
O'Meara GF, Vose FE, Carlson DB (1989) Environmental factors influencing oviposition by Culex (Culex) (Diptera: Culicidae) in two types of traps. J. Med. Entomol. 26:528–534. doi:10.1093/jmedent/26.6.528
Demari-Silva B, Suesdek L, Sallum MAM, Marrelli MT (2014) Wing geometry of Culex coronator (Diptera: Culicidae) from South and Southeast Brazil. Parasit. Vectors 7(1):1–9. doi:10.1186/1756-3305-7-174
Smith JP, Walsh JD, Cope EH, Tennant Jr RA, Kozak 3rd JA, Darsie Jr RF (2006) Culex coronator Dyar and Knab: a new Florida species record. J. Am. Mosq. Control Assoc. 22(2):330–332. doi:10.2987/8756-971X(2006)22[330:CCDAKA]2.0.CO;2
Darsie RF, Ward RA (2005) Identification and geographical distribution of the mosquitos of North America, North of Mexico. University Press of Florida, Gainsville
Yee D, Skiff J (2014) Interspecific competition of a new invasive mosquito, Culex coronator, and two container mosquitoes, Aedes albopictus and Cx. quinquefasciatus (Diptera: Culicidae), across different detritus environments. J. Med. Entomol. 51(1):89–96. doi:10.1603/ME13182
Connelly CR, Alto BW, O'Meara GF (2016) The spread of Culex coronator (Diptera: Culicidae) throughout Florida. J Vector Ecol. 41(1):195–199. doi:10.1111/jvec.12213
Alto BW, Connelly CR, O'Meara GF, Hickman D, Karr N (2014) Reproductive biology and susceptibility of Florida Culex coronator to infection with West Nile virus. Vector Borne Zoonotic Dis 14(8):606–614
Turell MJ, O'Guinn ML, Jones JW, Sardelis MR, Dohm DJ, Watts DM, Fernandez R, Travassos da Rosa A, Guzman H, Tesh R, Rossi CA, Ludwig V, Mangiafico JA, Kondig J, Wasieloski Jr LP, Pecor J, Zyzak M, Schoeler G, Mores CN, Calampa C, Lee JS, Klein TA (2005) Isolation of viruses from mosquitoes (Diptera: Culicidae) collected in the Amazon Basin region of Peru. J. Med. Entomol. 42(5):891–898. doi:10.1603/0022-2585(2005)042[0891:IOVFMD]2.0.CO;2
Goddard J, Varnado WC, Harrison BA (2006) Notes on the ecology of Culex coronator Dyar and Knab, in Mississippi. J. Am. Mosq. Control Assoc. 22(4):622–625. doi:10.2987/8756-971X(2006)22[622:NOTEOC]2.0.CO;2
Lawler S, Lanzaro GC (2005) Managing mosquitoes on the farm. University of California Division of Agriculture and Natural Resoruces Publications. 8158. http://anrcatalog.ucdavis.edu
Day JF, Shaman J (2009) Severe winter freezes enhance St. Louis encephalitis virus amplification and epidemic transmission in peninsular Florida. J. Med. Entomol. 46(6):1498–1506
Cutwa MM, O’Meara GF (2006) Photographic guide to common mosquitoes of Florida. Florida Medical Entomology Laboratory 1:1–83
Moran NA, Tran P, Gerardo NM (2005) Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Appl. Environ. Microbiol. 71(12):8802–8810. doi:10.1128/AEM.71.12.8802-8810.2005
Duguma D, Rugman-Jones P, Kaufman MG, Hall MW, Neufeld JD, Stouthamer R, Walton WE (2013) Bacterial communities associated with Culex mosquito larvae and two emergent aquatic plants of bioremediation importance. PLoS One 8(8):e72522. doi:10.1371/journal.pone.0072522
Zouache K, Raharimalala FN, Raquin V, Tran-Van V, Raveloson LH, Ravelonandro P, Mavingui P (2011) Bacterial diversity of field-caught mosquitoes, Aedes albopictus and Aedes aegypti, from different geographic regions of Madagascar. FEMS Microbiol. Ecol. 75(3):377–389. doi:10.1111/j.1574-6941.2010.01012.x
Kennedy K, Hall MW, Lynch MD, Moreno-Hagelsieb G, Neufeld JD (2014) Evaluating bias of Illumina-based bacterial 16S rRNA gene profiles. Appl. Environ. Microbiol. 80(18):5717–5722. doi:10.1128/AEM.01451-14
Bartram AK, Lynch MDJ, Stearns JC, Moreno-Hagelsieb G, Neufeld JD (2011) Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end Illumina reads. Appl. Environ. Microbiol. 77(11):3846–3852. doi:10.1128/AEM.02772-10
Ross AA, Neufeld JD (2015) Microbial biogeography of a university campus. Microbiome 3:66. doi:10.1186/s40168-015-0135-0
Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M (2014) Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS One 9 (8). doi: ARTN e105592
Lynch MDJ, Masella AP, Hall MW, Bartram AK, Neufeld JD (2013) AXIOME: automated exploration of microbial diversity. Giga Science 2:3. doi:10.1186/2047-217x-2-3
Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD (2012) PANDAseq: paired-end assembler for illumina sequences. BMC Bioinformatics 13:31. doi:10.1186/1471-2105-13-31
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10(10):996–998. doi:10.1038/nmeth.2604
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73(16):5261–5267. doi:10.1128/Aem.00062-07
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7(5):335–336. doi:10.1038/nmeth.f.303
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72(7):5069–5072. doi:10.1128/Aem.03006-05
Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, Oksanen MJ, Suggests M (2007) The vegan package. Community ecology package 10
Mielke PW, Berry KJ, Johnson ES (1976) Multi-response permutation procedures for a priori classifications. Commun Stat Theory Methods 5(14):1409–1424. doi:10.1080/03610927608827451
McCormick PV, Shuford RBE, Backus JG, Kennedy WC (1998) Spatial and seasonal patterns of periphyton biomass and productivity in the northern Everglades, Florida, USA. Hydrobiologia 362:185–208
Minard G, Tran FH, Van VT, Goubert C, Bellet C, Lambert G, Kim KL, Thuy TH, Mavingui P, Valiente Moro C (2015) French invasive Asian tiger mosquito populations harbor reduced bacterial microbiota and genetic diversity compared to Vietnamese autochthonous relatives. Front. Microbiol. 6:970. doi:10.3389/fmicb.2015.00970
Lu M, Hulcr J, Sun JH (2016) The role of symbiotic microbes in insect invasions. Annu Rev Ecol Evol S 47:487–505. doi:10.1146/annurev-ecolsys-121415-032050
Yurkov V, Stackebrandt E, Holmes A, Fuerst JA, Hugenholtz P, Golecki J, Gad'on N, Gorlenko VM, Kompantseva EI, Drews G (1994) Phylogenetic positions of novel aerobic, bacteriochlorophyll a-containing bacteria and description of Roseococcus thiosulfatophilus gen. nov., sp. nov., Erythromicrobium ramosum gen. nov., sp. nov., and Erythrobacter litoralis sp. nov. Int. J. Syst. Bacteriol. 44(3):427–434. doi:10.1099/00207713-44-3-427
Crotti E, Rizzi A, Chouaia B, Ricci I, Favia G, Alma A, Sacchi L, Bourtzis K, Mandrioli M, Cherif A, Bandi C, Daffonchio D (2010) Acetic acid bacteria, newly emerging symbionts of insects. Appl. Environ. Microbiol. 76(21):6963–6970. doi:10.1128/AEM.01336-10
Chandler J, Lang J, Bhatnagar S, Eisen J, Kopp A (2011) Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet. 7 (9). doi:10.1371/journal.pgen.1002272
Engel P, Moran NA (2013) The gut microbiota of insects—diversity in structure and function. FEMS Microbiol. Rev. 37(5):699–735. doi:10.1111/1574-6976.12025
Le PT, Pontarotti P, Raoult D (2014) Alphaproteobacteria species as a source and target of lateral sequence transfers. Trends Microbiol. 22(3):147–156. doi:10.1016/j.tim.2013.12.006
Alfonzo D, Grillet ME, Liria J, Navarro JC, Weaver SC, Barrera R (2005) Ecological characterization of the aquatic habitats of mosquitoes (Diptera: Culicidae) in enzootic foci of Venezuelan equine encephalitis virus in western Venezuela. J. Med. Entomol. 42(3):278–284
Herlemann DP, Lundin D, Labrenz M, Jurgens K, Zheng Z, Aspeborg H, Andersson AF (2013) Metagenomic de novo assembly of an aquatic representative of the verrucomicrobial class Spartobacteria. MBio 4(3):e00569–e00512. doi:10.1128/mBio.00569-12
Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald GF, Chater KF, van Sinderen D (2007) Genomics of Actinobacteria: tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 71(3):495–548. doi:10.1128/MMBR.00005-07
Johnson P, Azuolas J, Lavender CJ, Wishart E, Stinear TP, Hayman JA, Brown L, Jenkin GA, Fyfe J (2007) Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg. Infect. Dis. 13(11):1653–1660
Briones AM, Shililu J, Githure J, Novak R, Raskin L (2008) Thorsellia anophelis is the dominant bacterium in a Kenyan population of adult Anopheles gambiae mosquitoes. ISME J 2(1):74–82. doi:10.1038/ismej.2007.95
Lindh JM, Terenius O, Faye I (2005) 16S rRNA gene-based identification of midgut bacteria from field-caught Anopheles gambiae sensu lato and A. funestus mosquitoes reveals new species related to known insect symbionts. Appl. Environ. Microbiol. 71(11):7217–7223. doi:10.3201/eid1311.061369
Kaempfer P (2006) Thorsellia anophelis gen nov, sp nov, a new member of the Gammaproteobacteria. Int J Syst Evol Micr. 56:335–338. doi:10.1099/ijs.0.63999-0
Minard G, Mavingui P, Moro CV (2013) Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit. Vectors 6(1):1–12. doi:10.1186/1756-3305-6-146
Acknowledgements
We are grateful to Katja Engel and Arthur Domingos for their technical assistance. We thank Dr. Jonathan Day for his valuable comments on earlier version of the manuscript. JDN acknowledges a Discovery Grant from the Natural Sciences and Engineering Research Council (NSERC). DD was supported by funding from Florida Department of Agriculture and Consumer Services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(DOCX 376 kb)
Rights and permissions
About this article
Cite this article
Duguma, D., Hall, M.W., Smartt, C.T. et al. Temporal Variations of Microbiota Associated with the Immature Stages of Two Florida Culex Mosquito Vectors. Microb Ecol 74, 979–989 (2017). https://doi.org/10.1007/s00248-017-0988-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-0988-9