Abstract
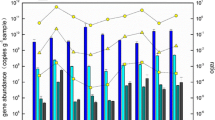
Changjiang Estuary, the largest estuary in China, encompasses a wide range of nutrient loading and trophic levels from the rivers to the sea, providing an ideal natural environment to explore relationships between functional diversity, physical/chemical complexity, and ecosystem function. In this study, molecular biological techniques were used to analyze the community structure and diversity of ammonia-oxidizing bacteria (AOB) in the sediments of Changjiang Estuary and its adjacent waters in East China Sea. The amoA gene (encoding ammonia monooxygenase subunit A) libraries analysis revealed extensive diversity within the β-Proteobacteria group of AOB, which were grouped into Nitrosospira-like and Nitrosomonas-like lineages. The majority of amoA gene sequences fell within Nitrosospira-like clade, and only a few sequences were clustered with the Nitrosomonas-like clade, indicating that Nitrosospira-like lineage may be more adaptable than Nitrosomonas-like lineage in this area. Multivariate statistical analysis indicated that the spatial distribution of the sedimentary β-Proteobacterial amoA genotype assemblages correlated significantly with nitrate, nitrite, and salinity. The vertical profile of amoA gene copies in gravity cores showed that intense sediment resuspension led to a deeper mixing layer. The horizontal distribution pattern of amoA gene copies was nearly correlated with the clayey mud belt in Changjiang Estuary and its adjacent area in East China Sea, where higher β-Proteobacteria phylogenetic diversity was observed. Meanwhile, those areas with high amoA copies in the surface sediments nearly matched those with low concentrations of dissolved oxygen and ammonium in the bottom water.








Similar content being viewed by others
References
Aller RC, Blair NE, Xia Q, Rude PD (1996) Remineralization rates, recycling and storage of carbon in Amazon shelf sediments. Cont Shelf Res 16:753–786
Álvarez-Salgado XA, Gilcoto M (2004) Inferring nitrification rates with an inverse method in a coastal upwelling system, Ría de Vigo (NW Spain). Mar Ecol Prog Ser 276:3–17
Araki N, Yamaguchi T, Yamazaki S, Harada H (2004) Quantification of amoA gene abundance and their amoA mRNA levels in activated sludge by real-time PCR. Water Sci Technol 50:1–8
Avrahami S, Jia Z, Neufeld JD, Murrell JC, Conrad R et al (2011) Active autotrophic ammonia-oxidizing bacteria in biofilm enrichments from simulated creek ecosystems at two ammonium concentrations respond to temperature manipulation. Appl Environ Microbiol 77(20):7329–7338
Bernhard AE, Donn T, Giblin AE, Stahl DA (2005) Loss of diversity of ammonia-oxidizing bacteria correlates with increasing salinity in an estuary system. Environ Microbiol 7:1289–1297
Bernhard AE, Tucker J, Giblin AE, Stahl DA (2007) Functionally distinct communities of ammonia-oxidizing bacteria along an estuarine salinity gradient. Environ Microbiol 9:1439–1447
Bianchi T, Mitra S, McKee B (2001) Sources of terrestrially derived organic carbon in lower Mississippi River and Louisiana Shelf sediments: implications for differential sedimentation and transport at the coastal margin. Mar Chem 77(2/3):211–223
Bianchi TS, Allison MA (2009) Large-river delta-front estuaries as natural “recorders” of global environmental change. PNAS 106:8085–8092
Bollmann A, Laanbroek HJ (2002) Influence of oxygen partial pressure and salinity on the community composition of ammonia-oxidizing bacteria in the Schelde Estuary. Aquat Microb Ecol 28:239–247
Cao H, Hong Y, Li M, Gu J (2011) Diversity and abundance of ammonia-oxidizing prokaryotes in sediments from the coastal Pearl River estuary to the South China Sea. Antonie Van Leeuwenhoek 100(4):545–556
Cao H, Hong Y, Li M, Gu J (2012) Community shift of ammonia-oxidizing bacteria along an anthropogenic pollution gradient from the Pearl River Delta to the South China Sea. Appl Microbiol Biotechnol 94(1):247–259
Carini SA, Joye SB (2008) Nitrification in Mono Lake, California: activity and community composition during contrasting hydrological regimes. Limnol Oceanogr 53:2546–2557
Cébron A, Coci M, Garnier J, Laanbroek HJ (2004) Denaturing gradient gel electrophoretic analysis of ammonia-oxidizing bacterial community structure in the lower Seine River: impact of Paris wastewater effluents. Appl Environ Microbiol 70:6726–6737
Chain P, Lamerdin J, Larimer F, Regala W, Lao V et al (2003) Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J Bacteriol 185:2759–2773
Coci M, Riechmann D, Bodelier P, Stefani S, Zwart G et al (2005) Effect of salinity on temporal and spatial dynamics of ammonia-oxidising bacteria from intertidal freshwater sediment. FEMS Microbiol Ecol 53:359–368
Dagg M, Benner R, Lohrenz S, Lawrence D (2004) Transformation of dissolved and particulate materials on continental shelves influenced by large rivers: plume processes. Cont Shelf Res 24:833–858
Dai Z, Du J, Zhang X, Su N, Li J (2011) Variation of riverine material loads and environmental consequences on the Changjiang (Yangtze) estuary in recent decades (1955–2008). Environ Sci Technol 45:223–227
Dang H, Li J, Chen R, Wang L, Guo L et al (2010) Diversity, abundance, and spatial distribution of sediment ammonia-oxidizing betaproteobacteria in response to environmental gradients and coastal eutrophication in Jiaozhou Bay, China. Appl Environ Microbiol 76:4691–4702
Dang H, Zhang X, Sun J, Li T, Zhang Z et al (2008) Diversity and spatial distribution of sediment ammonia-oxidizing crenarchaeota in response to estuarine and environmental gradients in the Changjiang Estuary and East China Sea. Microbiology 154:2084–2095
Dorador C, Busekow A, Vila I, Imhoff JF, Witzel KP (2008) Molecular analysis of enrichment cultures of ammonia oxidizers from the Salar de Huasco, a high altitude saline wetland in northern Chile. Extremophiles 12(3):405–414
Feng BW, Li XR, Wang JH, Hu ZY, Meng H et al (2009) Bacterial diversity of water and sediment in the Changjiang estuary and coastal area of the East China Sea. FEMS Microbiol Ecol 70:80–92
Francis CA, Beman JM, Kuypers MM (2007) New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation. ISME J 1:19–27
Francis CA, O'Mullan GD, Ward BB (2003) Diversity of ammonia monooxygenase (amoA) genes across environmental gradients in Chesapeake Bay sediments. Geobiology 1(2):129–140
Freitag TE, Chang L, Prosser JI (2006) Changes in the community structure and activity of betaproteobacterial ammonia-oxidizing sediment bacteria along a freshwater-marine gradient. Environ Microbiol 8:684–696
Galloway J, Dentener F, Capone D, Boyer E, Howarth R et al (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Garrity GM, Bell JA, Lilbum TG (2004) Taxonomic outline of the prokaryotes Bergey’s manual of systematic bacteriology, 2nd edn. Springer, Berlin
Gieseke A, Tarre S, Green M, de Beer D (2006) Nitrification in a biofilm at low pH values: role of in situ microenvironments and acid tolerance. Appl Environ Microbiol 72:4283–4292
Good IJ (1953) The population frequencies of species and the estimation of population parameters. Biometrika 40:237–264
Grommen R, Dauw L, Verstraete W (2005) Elevated salinity selects for a less diverse ammonia-oxidizing population in aquarium biofilters. FEMS Microbiol Ecol 52:1–11
Guo ZG, Yang ZS, Fan DJ, Pan YJ (2003) Seasonal variation of sedimentation in the Changjiang estuary mud area. J Geogr Sci 13:348–354
Guo Z, Lin T, Zhang G, Zheng M, Zhang Z et al (2007) The sedimentary fluxes of polycyclic aromatic hydrocarbons in the Yangtze River Estuary coastal sea for the past century. Sci Total Environ 386:33–41
Hill TC, Walsh KA, Harris JA, Moffett BF (2003) Using ecological diversity measures with bacterial communities. FEMS Microbiol Ecol 43:1–11
Hooper AB, Vannelli T, Bergmann DJ, Arciero DM (1997) Enzymology of the oxidation of ammonia to nitrite by bacteria. Antonie Van Leeuwenhoek 71:59–67
Hu L, Lin T, Shi X, Yang Z, Wang H et al (2011) The role of shelf mud depositional process and large river inputs on the fate of organochlorine pesticides in sediments of the Yellow and East China seas. Geophys Res Lett 38, L03602
Jenkins MC, Kemp WM (1984) The coupling of nitrification and denitrification in two estuarine sediments. Limnol Oceanogr 29:609–619
Jin T, Zhang T, Ye L, Lee OO, Wong YH et al (2011) Diversity and quantity of ammonia-oxidizing archaea and bacteria in sediment of the Pearl River Estuary, China. Appl Microbiol Biotechnol 90:1137–1145
Junier P, Kim OS, Hadas O, Imhoff JF, Witzel KP (2008) Evaluation of PCR primer selectivity and phylogenetic specificity by using amplification of 16S rRNA genes from betaproteobacterial ammonia-oxidizing bacteria in environmental samples. Appl Environ Microbiol 74:5231–5236
Junier P, Molina V, Dorador C, Hadas O, Kim OS et al (2010) Phylogenetic and functional marker gene to study ammonia-oxidizing microorganisms (AOM) in the environment. Appl Microbiol Biotechnol 85:425–440
Klotz MG, Alzerreca J, Norton JM (1997) A gene encoding a membrane protein exists upstream of the amoA/amoB genes in ammonia-oxidizing bacteria: a third member of the amo operon? FEMS Microbiol Lett 150:65–73
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55:485–529
Kuo DH, Robinson KG, Layton AC, Meyers AJ, Sayler GS (2010) Transcription levels (amoA mRNA-based) and population dominance (amoA gene-based) of ammonia-oxidizing bacteria. J Ind Microbiol Biotechnol 37:751–757
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
Li D, Daler D (2004) Ocean pollution from land-based sources: East China Sea, China. Ambio 33:107–113
Li D, Zhang J, Huang D, Wu Y, Liang J (2002) Oxygen depletion off the Changjiang (Yangtze River) Estuary. Sci China Ser D 45:1137–1146
Li J, Bai J, Gao HW, Liu G (2011) Distribution of ammonia-oxidizing betaproteobacteria community in surface sediment off the Changjiang River Estuary in summer. Acta Oceanol Sin 30(3):92–99
Li M, Xu K, Watanabe M, Chen Z (2007) Long-term variation in dissolved silicate, nitrogen, and phosphorus flux from the Yangtze River into the East China Sea and impacts on estuarine ecosystem. Estuar Coast Shelf Sci 71:3–12
Li S, Liu C, Li J, Liu X, Chetelat B et al (2010) Assessment of the sources of nitrate in the Changjiang River, China using a nitrogen and oxygen isotopic approach. Environ Sci Technol 44:1573–1578
Liu J, Li A, Xu K, Velozzi D, Yang Z et al (2006) Sedimentary features of the Yangtze River-derived along-shelf clinoform deposit in the East China Sea. Cont Shelf Res 26:2141–2156
Liu J, Xu K, Li A, Milliman J, Velozzi D et al (2007) Flux and fate of Yangtze River sediment delivered to the East China Sea. Geomorphology 85:208–224
Liu K, Peng T, Shaw P, Shiah F (2003) Circulation and biogeochemical processes in the East China Sea and the vicinity of Taiwan: an overview and a brief synthesis. Deep-Sea Res Pt II 50:1055–1064
Magalhães CM, Joye SB, Moreira RM, Wiebe WJ, Bordalo AA (2005) Effect of salinity and inorganic nitrogen concentrations on nitrification and denitrification rates in intertidal sediments and rocky biofilms of the Douro River estuary, Portugal. Water Res 39:1783–1794
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, New Jersey
McCaig AE, Embley TM, Prosser JI (1994) Molecular analysis of enrichment cultures of marine ammonia oxidizers. FEMS Microbiol Lett 120:363–367
Meade RH (1996) River-sediment inputs to major deltas. In: Milliman J, HAQ B (eds) Sea-level rise and coastal subsidence. Kluwer, London, pp 63–85
Mosier AC, Francis CA (2008) Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ Microbiol 10(11):3002–3016
Mullins TD, Britschigi TB, Krest RL, Giovannoni SJ (1995) Genetic comparisons reveal the same unknown bacterial lineages in Atlantic and Pacific bacterioplankton communities. Limnol Oceanogr 40:148–158
O’Mullan GD, Ward BB (2005) Relationship of temporal and spatial variabilities of ammonia-oxidizing bacteria to nitrification rates in Monterey Bay, CA. Appl Environ Microbiol 71:697–705
Pielou EC (1969) An introduction to mathematical ecology. Wiley-Interscience, New York
Poret-Peterson AT, Graham JE, Gulledge J, Klotz MG (2008) Transcription of nitrification genes by the methane-oxidizing bacterium, Methylococcus capsulatus strain Bath. ISME J 2:1213–1220
Prosser JI, Nicol GW (2008) Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ Microbiol 10:2931–2941
Purkhold U, Pommerening-Röser A, Juretschko S, Schmid MC, Koops HP et al (2000) Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implications for molecular diversity surveys. Appl Environ Microbiol 66:5368–5382
Purkhold U, Wagner M, Timmermann G, Pommerening-Röser A, Koops HP (2003) 16S rRNA and amoA-based phylogeny of 12 novel betaproteobacterial ammonia-oxidizing isolates: extension of the dataset and proposal of a new lineage within the nitrosomonads. Int J Syst Evol Microbiol 53(5):1485–1494
Radax R, Hoffmann F, Rapp HT, Leininger S, Schleper C (2012) Ammonia-oxidizing archaea as main drivers of nitrification in cold-water sponges. Environ Microbiol 14:909–923
Reigstad LJ, Richter A, Daims H, Urich T, Schwark L et al (2008) Nitrification in terrestrial hot springs of Iceland and Kamchatka. FEMS Microbiol Ecol 64:167–174
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Satoh H, Nakamura Y, Okabe S (2007) Influences of infaunal burrows on the community structure and activity of ammonia-oxidizing bacteria in intertidal sediments. Appl Environ Microbiol 73:1341–1348
Schloss PD, Handelsman J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71:1501–1506
Singleton DR, Furlong MA, Rathbu SL, Whitman WB (2001) Quantitative comparisons of 16S rRNA gene sequence libraries from environmental samples. Appl Environ Microbiol 67:4373–4376
Sinigalliano CD, Kuhn DN, Jones RD (1995) Amplification of the amoA gene from diverse species of ammonium-oxidizing bacteria and from an indigenous bacterial population from seawater. Appl Environ Microbiol 61:2702–2706
Sjoling S, Cowan DA (2003) High 16S rDNA bacterial diversity in glacial meltwater lake sediment, Bratina Island, Antarctica. Extremophiles 7:275–282
Stein LY, Arp DJ, Berube PM, Chain PS, Hauser L et al (2007) Whole-genome analysis of the ammonia-oxidizing bacterium, Nitrosomonas eutropha C91: implications for niche adaptation. Environ Microbiol 9(12):2993–3007
Stephen JR, Chang YJ, Macnaughton SJ, Kowalchuk GA, Leung KT et al (1999) Effect of toxic metals on indigenous soil β-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria. Appl Environ Microbiol 65:95–101
Tamura K, Peterson D, Peterson N, Stecher G, Nei M et al (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
ter Braak CJF, Šmilauer P (2002) CANOCO reference manual and CanoDraw for Windows user's guide: software for Canonical Community Ordination (version 4.5). Ithaca: Microcomputer Power
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The clustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Tu JB, Wang BD (2006) Assessment of eutrophication status in the Changjiang River Estuary and adjacent sea areas. Adv in Mar Sci 24:532–538, in Chinese, with English Summary
Urakawa H, Maki H, Kawabata S, Fujiwara T, Ando H et al (2006) Abundance and population structure of ammonia-oxidizing bacteria that inhabit canal sediments receiving effluents from municipal wastewater treatment plants. Appl Environ Microbiol 72:6845–6850
Wang B (2006) Cultural eutrophication in the Changjiang (Yangtze River) plume: history and perspective. Estuar Coast Shelf Sci 69:471–477
Wankel SD, Mosier AC, Hansel CM, Paytan A, Francis CA (2011) Spatial variability in nitrification rates and ammonia-oxidizing microbial communities in the agriculturally impacted Elkhorn Slough estuary, California. Appl Environ Microbiol 77(1):269–280
Ward BB, Eveillard D, Kirshtein JD, Nelson JD, Voytek MA et al (2007) Ammonia-oxidizing bacterial community composition in estuarine and oceanic environments assessed using a functional gene microarray. Environ Microbiol 9:2522–2538
Warrington R (1878) On nitrification. J Chem Soc 33:44–51
Wuchter C, Abbas B, Coolen MJL, Herfort L, van Bleijswijk J et al (2006) Archaeal nitrification in the ocean. PNAS 103:12317–12322
Yang Z, Wang H, Saito Y, Milliman JD, Xu K et al (2006) Dam impacts on the Changjiang (Yangtze) River sediment discharge to the sea: the past 55 years and after the Three Gorges Dam. Water Resour Res 42, W04407
Yool A, Martin AP, Fernández C, Clark DR (2007) The significance of nitrification for oceanic new production. Nature 447:999–1002
Zhang Y, Jiao N (2007) Dynamics of aerobic anoxygenic phototrophic bacteria in the East China Sea. FEMS Microbiol Ecol 61:459–469
Zheng Y, Hou L, Liu M, Lu M, Zhao H et al (2012) Diversity, abundance, and activity of ammonia-oxidizing bacteria and archaea in Chongming eastern intertidal sediments. Appl Microbiol Biotechnol. doi:10.1007/s00253-012-4512-3
Zhou M, Yan T, Zou JZ (2003) Preliminary analysis of the characteristics of red tide areas in Changjiang River Estuary and its adjacent sea. Chin J Appl Ecol 14:1031–1038, in Chinese, with English summary
Acknowledgments
We thank Mr. Guodong Chen for analyzing dissolved N, P, and Si concentrations. We are also grateful to Prof. Guanpin Yang from the Ocean University of China for kind suggestions. The endeavors of all staff on R/V Run-Jiang for assistance with the collection of samples and geochemical data during the cruise are gratefully acknowledged. This work was supported by the National Basic Research Program of China (973 Program) (No. 2011CB403602), the National Natural Science Foundation of China (Nos. 40920164004 and 41221004) and the National Marine Public Welfare Research Project (201205031). The authors would like to thank the editor and anonymous reviewers for their valuable comments and suggestions to improve the quality of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Zhen, Y., He, H. et al. Diversity, Abundance, and Spatial Distribution of Ammonia-Oxidizing β-Proteobacteria in Sediments from Changjiang Estuary and Its Adjacent Area in East China Sea. Microb Ecol 67, 788–803 (2014). https://doi.org/10.1007/s00248-013-0341-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-013-0341-x