Abstract
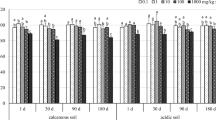
A soil sterilization–reinoculation approach was used to manipulate soil microbial diversity and to assess the effect of the diversity of the ammonia-oxidizing bacteria (AOB) on the recovery of the nitrifying community to metal stress (zinc). Gamma-irradiated soil was inoculated with 13 different combinations of up to 22 different soils collected worldwide to create varying degrees of AOB diversity. Two months after inoculation, AOB amoA DGGE based diversity (weighted richness) varied more than 10-fold among the 13 treatments, the largest value observed where the number of inocula had been largest. Subsequently, the 13 treatments were either or not amended with ZnCl2. Initially, Zn amendment completely inhibited nitrification. After 6 months of Zn exposure, recovery of the potential nitrification activity in the Zn amended soils ranged from <10 % to >100 % of the potential nitrification activity in the corresponding non-amended soils. This recovery was neither related to DGGE-based indices of AOB diversity nor to the AOB abundance assessed 2 months after inoculation (p > 0.05). However, recovery was significantly related (r = 0.75) to the potential nitrification rate before Zn amendment and only weakly to the number of soil inocula used in the treatments (r = 0.46). The lack of clear effects of AOB diversity on recovery may be related to an inherently sufficient diversity and functional redundancy of AOB communities in soil. Our data indicate that potential microbial activity can be a significant factor in recovery.


Similar content being viewed by others
References
Konopka A (2009) What is microbial community ecology? ISME 3:1223–1230
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, Tilman D, Wardle DA (2001) Ecology — biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294:804–808
Girvan MS, Campbell CD, Killham K, Prosser JI, Glover LA (2005) Bacterial diversity promotes community stability and functional resilience after perturbation. Env Microbiol 7:301–313
Griffiths BS, Ritz K, Bardgett RD, Cook R, Christensen S, Ekelund F, Sorensen SJ, Bååth E, Bloem J, de Ruiter PC, Dolfing J, Nicolardot B (2000) Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions: an examination of the biodiversity-ecosystem function relationship. Oikos 90:279–294
Griffiths BS, Bonkowski M, Roy J, Ritz K (2001) Functional stability, substrate utilisation and biological indicators of soils following environmental impacts. Appl Soil Ecol 16:49–61
Wertz S, Degrange V, Prosser JI, Poly F, Commeaux C, Guillaumaud N, Le Roux X (2007) Decline of soil microbial diversity does not influence the resistance and resilience of key soil microbial functional groups following a model disturbance. Env Microbiol 9:2211–2219
Griffiths BS, Ritz K, Wheatley R, Kuan HL, Boag B, Christensen S, Ekelund F, Sorensen SJ, Muller S, Bloem J (2001) An examination of the biodiversity–ecosystem function relationship in arable soil microbial communities. Soil Biol Biochem 33:1713–1722
Naeem S, Li SB (1997) Biodiversity enhances ecosystem reliability. Nature 390:507–509
Griffiths BS, Hallett PD, Kuan HL, Gregory AS, Watts CW, Whitmore AP (2008) Functional resilience of soil microbial communities depends on both soil structure and microbial community composition. Biol Fertil Soils 44:745–754
Marschner P, Rumberger A (2004) Rapid changes in the rhizosphere bacterial community structure during re-colonization of sterilized soil. Biol Fertil Soils 40:1–6
Díaz-Raviña M, Bååth E (2001) Response of soil bacterial communities pre-exposed to different metals and reinoculated in an unpolluted soil. Soil Biol Biochem 33:241–248
Fait G, Broos K, Zrna S, Lombi E, Hamon R (2006) Tolerance of nitrifying bacteria to copper and nickel. Environ Toxicol Chem 25:2000–2005
Rusk JA, Hamon RE, Stevens DP, McLaughlin MJ (2004) Adaptation of soil biological nitrification to heavy metals. Environ Sci Technol 38:3092–3097
Smolders E, Oorts K, van Sprang P, Schoeters I, Janssen CR, McGrath SP, McLaughlin MJ (2009) Toxicity of trace metals in soil as affected by soil type and aging after contamination: using calibrated bioavailability models to set ecological soil standards. Environ Toxicol Chem 28:1633–1642
Mertens J, Springael D, De Troyer I, Cheyns K, Wattiau P, Smolders E (2006) Long-term exposure to elevated zinc concentrations induced structural changes and zinc tolerance of the nitrifying community in soil. Env Microbiol 8:2170–2178
Ruyters S, Mertens J, Springael D, Smolders E (2010) Stimulated activity of the soil nitrifying community accelerates community adaptation to Zn stress. Soil Biol Biochem 42:766–772
Witter E, Gong P, Bååth E, Marstorp H (2000) A study of the structure and metal tolerance of the soil microbial community six years after cessation of sewage sludge applications. Environ Toxicol Chem 19:1983–1991
Díaz-Raviña M, Bååth E (1996) Development of metal tolerance in soil bacterial communities exposed to experimentally increased metal levels. Appl Environ Microbiol 62:2970–2977
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Prosser JI, Nicol GW (2012) Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol 20:523–531
Mertens J, Broos K, Wakelin SA, Kowalchuk GA, Springael D, Smolders E (2009) Bacteria, not archaea, restore nitrification in a zinc-contaminated soil. ISME 3:916–923
Smolders E, Brans K, Coppens F, Merckx R (2001) Potential nitrification rate as a tool for screening toxicity in metal-contaminated soils. Environ Toxicol Chem 20:2469–2474
Trevors JT (1996) Sterilization and inhibition of microbial activity in soil. J Microbiol Methods 26:53–59
Oorts K, Bronckaers H, Smolders E (2006) Discrepancy of the microbial response to elevated copper between freshly spiked and long-term contaminated soils. Environ Toxicol Chem 25:845–853
Doelman P, Haanstra L (1989) Short-term and long-term effects of heavy-metals on phosphatase-activity in soils — an ecological dose–response model approach. Biol Fertil Soils 8:235–241
Purkhold U, Pommerening-Roser A, Juretschko S, Schmid MC, Koops HP, Wagner M (2000) Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implications for molecular diversity surveys. Appl Environ Microbiol 66:5368–5382
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55:485–529
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Norton JM, Alzerreca JJ, Suwa Y, Klotz MG (2002) Diversity of ammonia monooxygenase operon in autotrophic ammonia-oxidizing bacteria. Arch Microbiol 177:139–149
Stephen JR, Kowalchuk GA, Bruns MAV, McCaig AE, Phillips CJ, Embley TM, Prosser JI (1998) Analysis of beta-subgroup proteobacterial ammonia oxidizer populations in soil by denaturing gradient gel electrophoresis analysis and hierarchical phylogenetic probing. Appl Environ Microbiol 64:2958–2965
Mertens B, Boon N, Verstraete W (2005) Stereospecific effect of hexachlorocyclohexane on activity and structure of soil methanotrophic communities. Env Microbiol 7:660–669
Marzorati M, Wittebolle L, Boon N, Daffonchio D, Verstraete W (2008) How to get more out of molecular fingerprints: practical tools for microbial ecology. Env Microbiol 10:1571–1581
Ramette A (2007) Multivariate analyses in microbial ecology. FEMS Microbiol Ecol 62:142–160
Nicol GW, Schleper C (2006) Ammonia-oxidising Crenarchaeota: important players in the nitrogen cycle? Trends Microbiol 14:207–212
Okano Y, Hristova KR, Leutenegger CM, Jackson LE, Denison RF, Gebreyesus B, Lebauer D, Scow KM (2004) Application of real-time PCR to study effects of ammonium on population size of ammonia-oxidizing bacteria in soil. Appl Environ Microbiol 70:1008–1016
Jia ZJ, Conrad R (2009) Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Env Microbiol 11:1658–1671
Zhang LM, Offre PR, He JZ, Verhamme DT, Nicol GW, Prosser JI (2010) Autotrophic ammonia oxidation by soil thaumarchaea. Proc Natl Acad Sci U S A 107:17240–17245
Ruyters S, Nicol GW, Prosser JI, Lievens B, Smolders E (2013) Activity of the ammonia oxidising bacteria is responsible for zinc tolerance development of the ammonia oxidising community in soil: a stable isotope probing study. Soil Biol Biochem 58:244–247
Acknowledgments
This work was financially supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen) and by a post-doctoral mandate of the KULeuven (PDM-KULeuven). The authors thank Dr. Jelle Mertens for help with the experimental design.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruyters, S., Springael, D. & Smolders, E. Recovery of Soil Ammonia Oxidation After Long-Term Zinc Exposure Is Not Related to the Richness of the Bacterial Nitrifying Community. Microb Ecol 66, 312–321 (2013). https://doi.org/10.1007/s00248-013-0210-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-013-0210-7