Abstract
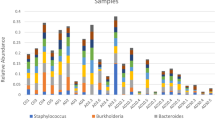
Bacterial disease is a significant issue for larviculture of several species of shellfish, including oysters. One source of bacteria is the seawater used throughout the hatchery. In this study carried out at a commercial oyster hatchery in Tasmania, Australia, the diversity of the bacterial community and its relationship with larval production outcomes were studied over a 2-year period using terminal restriction fragment length polymorphism and tag-encoded pyrosequencing. The bacterial communities were very diverse, dominated by the Alphaproteobacteria, Gammaproteobacteria, Flavobacteria and Cyanobacteria. The communities were highly variable on scales of days, weeks and seasons. The difference between the intake seawater and treated clean seawater used in the hatchery was smaller than the observed temporal differences in the seawater throughout the year. No clear correlation was observed between production outcomes and the overall bacterial community structure. However, one group of Cyanobacterial sequences was more abundant when mass mortality events occurred than when healthy spat were produced although they were always present.






Similar content being viewed by others
References
Paillard C, Le Roux F, Borreg JJ (2004) Bacterial disease in marine bivalves, a review of recent studies: trends and evolution. Aquat Living Resour 17:477–498
Prado S, Romalde JL, Montes J, Barja JL (2005) Pathogenic bacteria isolated from disease outbreaks in shellfish hatcheries. First description of Vibrio neptunius as an oyster pathogen. Dis Aquat Org 67:209–215
Bourne DG, Young N, Webster N, Payne M, Salmon M, Demel S, Hall M (2004) Microbial community dynamics in a larval aquaculture system of the tropical rock lobster, Panulirus ornatus. Aquaculture 242:31–51
Verner-Jeffreys DW, Shields RJ, Bricknell IR, Birkbeck TH (2004) Effects of different water treatment methods and antibiotic addition on larval survival and gut microflora development in Atlantic halibut (Hippoglossus hippoglossus L.) yolk-sac larvae. Aquaculture 232:129–143
Altug G, Cardak M, Ciftci PS, Gurun S (2010) The application of viable count procedures for measuring viable cells in the various marine environments. J Appl Microbiol 108:88–95
Kogure K, Simidu U, Taga N, Colwell RR (1987) Correlation of direct viable counts with heterotrophic activity for marine bacteria. Appl Environ Microbiol 53:2332–2337
Pfeffer C, Oliver JD (2003) A comparison of thiosulphate-citrate-bile salts-sucrose (TCBS) agar and thiosulphate-chloride-iodide (TCI) agar for the isolation of Vibrio species from estuarine environments. Lett Appl Microbiol 36:150–151
Andersson AF, Riemann L, Bertilsson S (2010) Pyrosequencing reveals contrasting seasonal dynamics of taxa within Baltic Sea bacterioplankton communities. ISME J 4:171–181
Gilbert JA, Field D, Swift P, Thomas S, Cummings D, Temperton B, Weynberg K, Huse S, Hughes M, Joint I, Somerfield PJ, Muehling M (2010) The taxonomic and functional diversity of microbes at a temperate coastal site: a 'multi-omic' study of seasonal and diel temporal variation. Plos One 5:e15545. doi:10.1371/journal.pone.0015545
Treusch AH, Vergin KL, Finlay LA, Donatz MG, Burton RM, Carlson CA, Giovannoni SJ (2009) Seasonality and vertical structure of microbial communities in an ocean gyre. ISME J 3:1148–1163
Kirchman DL, Cottrell MT, Lovejoy C (2010) The structure of bacterial communities in the western Arctic Ocean as revealed by pyrosequencing of 16S rRNA genes. Environ Microbiol 12:1132–1143
Fuhrman JA, Hewson I, Schwalbach MS, Steele JA, Brown MV, Naeem S (2006) Annually reoccurring bacterial communities are predictable from ocean conditions. Proc Natl Acad Sci U S A 103:13104–13109
Hill PG, Heywood JL, Holland RJ, Purdie DA, Fuchs BM, Zubkov MV (2012) Internal and external influences on near-surface microbial community structure in the vicinity of the Cape Verde islands. Microb Ecol 63:139–148
Salvesen I, Skjermo J, Vadstein O (1999) Growth of turbot (Scophthalmus maximus L.) during first feeding in relation to the proportion of r/K-strategists in the bacterial community of the rearing water. Aquaculture 175:337–350
Skjermo J, Vadstein O (1999) Techniques for microbial control in the intensive rearing of marine larvae. Aquaculture 177:333–343
van der Meeren T, Brunvold L, Sandaa RA, Bergh O, Castberg T, Thyrhaug R, Mangor-Jensen A (2011) Water quality and microbial community structure in juvenile Atlantic cod (Gadus morhua L.) cultures. Aquaculture 316:111–120
Dowd SE, Callaway TR, Wolcott RD, Sun Y, McKeehan T, Hagevoort RG, Edrington TS (2008) Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol 8:125–133
Wolcott RD, Gontcharova V, Sun Y, Dowd SE (2009) Evaluation of the bacterial diversity among and within individual venous leg ulcers using bacterial tag-encoded FLX and titanium amplicon pyrosequencing and metagenomic approaches. BMC Microbiol 9:226–237
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Quince C, Lanzen A, Curtis TP, Davenport RJ, Hall N, Head IM, Read LF, Sloan WT (2009) Accurate determination of microbial diversity from 454 pyrosequencing data. Nat Meth 6(9):639–641
Gontcharova V, Youn E, Wolcott RD, Hollister EB, Gentry TJ, Dowd SE (2010) Black box chimera check (B2C2): a Windows-based software for batch depletion of chimeras from bacterial 16S rRNA gene datasets. Open Microbiol J 4:47–52
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM (2009) The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:D141–D145
Kunin V, Engelbrektson A, Ochman H, Hugenholtz P (2010) Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ Microbiol 12:118–123
Zhou J, Wu L, Deng Y, Zhi X, Jiang Y-H, Tu Q, Xie J, Van Nostrand JD, He Z, Yang Y (2011) Reproducibility and quantitation of amplicon sequencing-based detection. ISME J 5:1303–1313
Gilbert JA, Steele JA, Caporaso JG, Steinbruck L, Reeder J, Temperton B, Huse S, McHardy AC, Knight R, Joint I, Somerfield P, Fuhrman JA, Field D (2012) Defining seasonal marine microbial community dynamics. ISME J 6:298–308
Schattenhofer M, Fuchs BM, Amann R, Zubkov MV, Tarran GA, Pernthaler J (2009) Latitudinal distribution of prokaryotic picoplankton populations in the Atlantic Ocean. Environ Microbiol 11:2078–2093
Jorquera MA, Lody M, Leyton Y, Riquelme C (2004) Bacteria of subclass gamma-proteobacteria associated with commercial Argopecten purpuratus (Lamark, 1819) hatcheries in Chile. Aquaculture 236:37–51
Nakase G, Nakagawa Y, Miyashita S, Nasu T, Senoo S, Matsubara H, Eguchi M (2007) Association between bacterial community structures and mortality of fish larvae in intensive rearing systems. Fish Sci 73:784–791
Asplund ME, Rehnstam-Holm A-S, Atnur V, Raghunath P, Saravanan V, Harnstrom K, Collin B, Karunasagar I, Godhe A (2011) Water column dynamics of Vibrio in relation to phytoplankton community composition and environmental conditions in a tropical coastal area. Environ Microbiol 13:2738–2751
Martins R, Fernandez N, Beiras R, Vasconcelos V (2007) Toxicity assessment of crude and partially purified extracts of marine Synechocystis and Synechococcus cyanobacterial strains in marine invertebrates. Toxicon 50:791–799
Frazao B, Martins R, Vasconcelos V (2010) Are known cyanotoxins involved in the toxicity of picoplanktonic and filamentous North Atlantic marine Cyanobacteria? Mar Drugs 8:1908–1919
Olafsen JA (2001) Interactions between fish larvae and bacteria in marine aquaculture. Aquaculture 200:223–247
Acknowledgments
The authors would like to thank the staff of Shellfish Culture Ltd, particularly those at the Bicheno hatchery, for their assistance with sampling. We would also like to thank Adam Smolenski at the Central Science Laboratory (University of Tasmania) for running the fragment analyses.
This work formed part of a project of the Australian Seafood Cooperative Research Centre and received funds from the Australian Government’s CRC programme, the Fisheries R&D Corporation and other CRC participants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Powell, S.M., Chapman, C.C., Bermudes, M. et al. Dynamics of Seawater Bacterial Communities in a Shellfish Hatchery. Microb Ecol 66, 245–256 (2013). https://doi.org/10.1007/s00248-013-0183-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-013-0183-6