Abstract
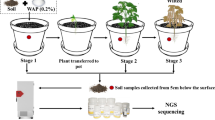
Mine wastes have been considered as a source of heavy metal (HM) contamination in the environment and negatively impact many important ecosystem services provided by soils. Plants like Miscanthus, which tolerate high HM concentrations in soil, are often used for phytoremediation and provide the possibility to use these soils at least for the production of energy crops. However, it is not clear if plant growth at these sites is limited by the availability of nutrients, mainly nitrogen, as microbes in soil might be affected by the contaminant. Therefore, in this study, we investigated in a greenhouse experiment the response of ammonia-oxidizing microbes in the root–rhizosphere complex of Miscanthus × giganteus grown in soils with different levels of long-term arsenic (As) and lead (Pb) contamination. Quantitative PCR of the ammonia monooxigenease gene (amoA) was performed to assess the abundance of ammonia-oxidizing bacteria (AOB) and archaea (AOA) at two different points of plant growth. Furthermore, bulk soil samples before planting were analyzed. In addition, terminal restriction fragment length polymorphism (T-RFLP) analysis was used to investigate the diversity of archaeal amoA amplicons. Whereas high concentrations of As and Pb in soil (83 and 15 g/kg, respectively) resulted independent from plant growth in a clear reduction of AOA and AOB compared to the control soils with lower HM contents, in soils with contamination levels of 10 g/kg As and 0.2 g/kg Pb, only AOB were negatively affected in bulk soil samples. Diversity analysis of archaeal amoA genes revealed clear differences in T-RFLP patterns in response to the degree of HM contamination. Therefore, our results could clearly prove the different response patterns of AOA and AOB in HM-contaminated soils and the development of archaeal amoA phylotypes which are more tolerant towards HMs in soil samples from the areas that were impacted the most by mining waste, which could contribute to functional redundancy of ammonia-oxidizing microbes in soils and stability of nitrification pattern.



Similar content being viewed by others
References
Alloway BJ (1990) Heavy metals in soils. Blackie, Glasgow
Chaney RL, Malik M, Li YM, Brown SL, Brewer EP, Angle JS, Baker AJM (1997) Phytoremediation of soil metals. Curr Opin Biotechnol 8:279–284
Vangronsveld J, Herzig R, Weyens N, Boulet J, Adriaensen K, Ruttens A, Thewys T, Vassilev A, Meers E, Nehnevajova E, van der Lelie D, Mench M (2009) Phytoremediation of contaminated soils and groundwater: lessons from the field. Environ Sci Pollut Res 16:765–794
Tordoff GM, Baker AJM, Willis AJ (2000) Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 41:219–228
Dalzell DJB, Alte S, Aspichueta E, de la Sota A, Etxebarria J, Gutierrez M, Hoffmann CC, Sales D, Obst U, Christofi N (2002) A comparison of five rapid direct toxicity assessment methods to determine toxicity of pollutants to activated sludge. Chemosphere 47:535–545
Broos K, Mertens J, Smolders E (2005) Toxicity of heavy metals in soil assessed with various soil microbial and plant growth assays: as comparative study. Environ Toxicol Chem 24:634–640
Verstraete W, Mertens B (2004) The key role of soil microbes. In: Doelman P, Eijsackers HJP (eds) Vital soil: function, value, and properties, vol 29. Elsevier, Amsterdam, pp 127–157
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55:485–529
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Prosser JI (1989) Autotrophic nitrification in bacteria. Adv Microb Physiol 30:125–181
Stephen JR, Chang Y-J, Macnaughton SJ, Kowalchuk GA, Leung KT, Flemming CA, White DC (1999) Effect of toxic metals on indigenous soil beta-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria. Appl Environ Microbiol 65:95–101
Gremion F, Chatzinotas A, Kaufmann K, Von Sigler W, Harms H (2004) Impacts of heavy metal contamination and phytoremediation on a microbial community during a twelve-month microcosm experiment. FEMS Microbiol Ecol 48:273–283
Mertens J, Springael D, De Troyer I, Cheyns K, Wattiau P, Smolders E (2006) Long-term exposure to elevated zinc concentrations induced structural changes and zinc tolerance of the nitrifying community in soil. Environ Microbiol 8:2170–2178
Schleper C, Jurgens G, Jonuscheit M (2005) Genomic studies of uncultivated archaea. Nat Rev Microbiol 3:479–488
Valentine DL (2007) Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat Rev Microbiol 5:316–323
Ruyters S, Mertens J, Springael D, Smolders E (2010) Stimulated activity of the soil nitrifying community accelerates community adaptation to Zn stress. Soil Biol Biochem 42:766–772
Xia Y, Zhu Y-G, Gu Q, He J-Z (2007) Does long-term fertilization treatment affect the response of soil ammonia-oxidizing bacterial communities to Zn contamination? Plant Soil 301:245–254
Mertens J, Broos K, Wakelin SA, Kowalchuk GA, Springael D, Smolders E (2009) Bacteria, not archaea, restore nitrification in a zinc-contaminated soil. ISME J 3:916–923
Mertens J, Wakelin SA, Broos K, McLaughlin MJ, Smolders E (2010) Extent of copper tolerance and consequences for functional stability of the ammonia-oxidizing community in long-term copper-contaminated soils. Environ Toxicol Chem 29:27–37
Frey B, Pesaro M, Rüdt A, Widmer F (2008) Resilience of the rhizosphere Pseudomonas and ammonia-oxidizing bacterial populations during phytoextraction of heavy metal polluted soil with poplar. Environ Microbiol 10:1433–1449
Li XF, Zhu YG, Cavagnaro TR, Chen MM, Sun JW, Chen XP, Qiao M (2009) Do ammonia-oxidizing archaea respond to soil Cu contamination similarly as ammonia-oxidizing bacteria? Plant Soil 324:209–217
Diaz-Ravina M, Baath E (1996) Development of metal tolerance in soil bacterial communities exposed to experimentally increased metal levels. Appl Environ Microbiol 62:2970–2977
Giller KE, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30:1389–1414
Collins RE, Rocap G (2007) REPK: an analytical web server to select restriction endonucleases for terminal restriction fragment length polymorphism analysis. Nucleic Acids Res 35:W58–W62
Lueders T, Kindler R, Miltner A, Friedrich MW, Kaestner M (2006) Identification of bacterial micropredators distinctively active in a soil microbial food web. Appl Environ Microbiol 72:5342–5348
Culman SW, Bukowski R, Gauch HG, Cadillo-Quiroz H, Buckley DH (2009) T-REX: software for the processing and analysis of T-RFLP data. BMC Bioinforma 10:10
Thioulouse J, Chessel D, Doledec S, Olivier JM (1997) ADE-4: a multivariate analysis and graphical display software. Stat Comput 7:75–83
Hommel G (1988) A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 75:383–386
Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10:2966–2978
Schauss K, Focks A, Leininger S, Kotzerke A, Heuer H, Thiele-Bruhn S, Sharma S, Wilke BM, Matthies M, Smalla K, Munch JC, Amelung W, Kaupenjohann M, Schloter M, Schleper C (2009) Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils. Environ Microbiol 11:446–456
Cui Y, Du X, Weng L, Van Riemsdijk WH (2010) Assessment of in situ immobilization of lead (Pb) and arsenic (As) in contaminated soils with phosphate and iron: solubility and bioaccessibility. Water Air Soil Pollut 213:95–104
Giller KE, Witter E, McGrath SP (2009) Heavy metals and soil microbes. Soil Biol Biochem 41:2031–2037
Haferburg G, Kothe E (2007) Microbes and metals: interactions in the environment. J Basic Microbiol 47:453–467
Park S, Ely RL (2008) Genome-wide transcriptional responses of Nitrosomonas europaea to zinc. Arch Microbiol 189:541–548
Tourna M, Stieglmeier M, Spang A, Konneke M, Schintlmeister A, Urich T, Engel M, Schloter M, Wagner M, Richter A, Schleper C (2011) Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci U S A 108:8420–8425
Gubry-Rangin C, Nicol GW, Prosser JI (2010) Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol Ecol 74:566–574
De Boer W, Kowalchuk GA (2001) Nitrification in acid soils: micro-organisms and mechanisms. Soil Biol Biochem 33:853–866
Lehtovirta LE, Prosser JI, Nicol GW (2009) Soil pH regulates the abundance and diversity of Group 1.1c Crenarchaeota. FEMS Microbiol Ecol 70:367–376
Nugroho RA, Roling WFM, Laverman AM, Verhoef HA (2007) Low nitrification rates in acid scots pine forest soils are due to pH-related factors. Microb Ecol 53:89–97
Yao H, Gao Y, Nicol GW, Campbell CD, Prosser JI, Zhang L, Han W, Singh BK (2011) Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl Environ Microbiol 77:4618–4625
Brimecomb MJ, de Leij FA, Lynch JM (2001) The effect of root exudates on rhizosphere microbial populations. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere: biochemistry and organic substances at the soil–plant interface. Marcel Dekker, New York, pp 95–140
Marschner P, Yang CH, Lieberei R, Crowley DE (2001) Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol Biochem 33:1437–1445
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461:976–U234
Ollivier J, Töwe S, Bannert A, Hai B, Kastl E-M, Meyer A, Su MX, Kleineidam K, Schloter M (2011) Nitrogen turnover in soil and global change. FEMS Microbiol Ecol 78:3–16
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ollivier, J., Wanat, N., Austruy, A. et al. Abundance and Diversity of Ammonia-Oxidizing Prokaryotes in the Root–Rhizosphere Complex of Miscanthus × giganteus Grown in Heavy Metal-Contaminated Soils. Microb Ecol 64, 1038–1046 (2012). https://doi.org/10.1007/s00248-012-0078-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-012-0078-y