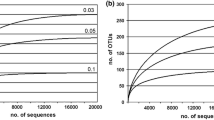
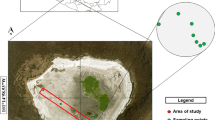
Abstract
Mining of pyrite minerals is a major environmental issue involving both biological and geochemical processes. Here we present a study of an artificial lake of a former uranium open pit mine with the aim to connect the chemistry and bacterial community composition (454-pyrosequencing of 16S rRNA genes) in the stratified water column. A shift in the water chemistry from oxic conditions in the epilimnion to anoxic, alkaline, and metal and sulfide-rich conditions in the hypolimnion was corresponded by a strong shift in the bacterial community, with few shared operational taxonomic units (OTU) between the water layers. The epilimnetic bacterial community of the lake (∼20 years old) showed similarities to other temperate freshwater lakes, while the hypolimnetic bacterial community showed similarity to extreme chemical environments. The epilimnetic bacterial community had dominance of Actinobacteria and Betaproteobacteria. The hypolimnion displayed a higher bacterial diversity and was dominated by the phototrophic green sulphur bacterium of the genus Chlorobium (ca. 40 % of the total community). Deltaproteobacteria were only represented in the hypolimnion and the most abundant OTUs were affiliated with ferric iron and sulfate reducers of the genus Geobacter and Desulfobulbus, respectively. The chemistry is clearly controlling, especially the hypolimnetic, bacterial community but the community composition also indicates that the bacteria are involved in metal cycling in the lake.






Similar content being viewed by others
References
Andersson A, Dahlman B, Gee DG, Snäll S (1985) The scandinavian alum shales. Rep. Geological Survey of Sweden NR 56
Baker BJ, Banfield JF (2003) Microbial communities in acid mine drainage. FEMS Microbiol Ecol 44:139–152
Beller HR (2005) Anaerobic, nitrate-dependent oxidation of U(IV) oxide minerals by the chemolithoautotrophic bacterium Thiobacillus denitrificans. Appl Environ Microbiol 71:2170–2174
Börjesson E (2002) Natural treatment system for heavy metal drainage: a pilot-scale study at the Ranstad filed site, Sweden. Rep. ISBN 91-7668-303-6, Örebro University, Örebro University
Brooks SC, Fredrickson JK, Carrol SL, Kennedy DW, Zachara JM et al (2003) Inhihition of bacterial U(VI) reduction by calcium. Environ Sci Technol 37:1850–1858
Byden S, Larsson A-M, Olsson M (2003) Mäta vatten, undersökning av sött och salt vatten. University of Göteborg
Davison W (1993) Iron and manganese in lakes. Earth Sci Rev 34:119–163
Drever JI (1997) Heavy metals and metalloids. In the geochemistry of natural waters surface and groundwater environments. McCormack P (ed) Prentice-Hall, Inc, NJ, pp 175–196
Eiler A, Bertilsson S (2004) Composition of freshwater bacterial communities associated with cyanobacterial blooms in four Swedish lakes. Environ Microbiol 6:1228–1243
Finneran KT, Housewright ME, Lovely DR (2002) Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ Microbiol 4:510–516
Gadd GM (2010) Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 156:609–643
Gihring TM, Green SJ, Schad CW (2011) Massively parallel rRNA gene sequencing exacerbates the potential for biased community diversity comparisons due to variable library sizes. Environ Microbiol. doi:10.1111/j.1462-2920.2011.02550.x:
Glockner FO, Zaichikov E, Belkova N, Denissova L, Pernthaler J et al (2000) Comparative 16S rRNA analysis of lake bacterioplankton reveals globally distributed phylogenetic clusters including an abundant group of actinobacteria. Appl Environ Microbiol 66:5053–5065
Gregersen LH, Habicht KS, Peduzzi S, Tonolla M, Canfield DE et al (2009) Dominance of a clonal green sulfur bacterial population in a stratified lake. FEMS Microbiol Ecol 70:30–41
Hahn MW (2009) Description of seven candidate species affiliated with the phylum Actinobacteria, representing planktonic freshwater bacteria. Int J Syst Evol Microbiol 59:112–117
Halm H, Musat N, Lam P, Langlois R, Musat F et al (2009) Co-occurrence of denitrification and nitrogen fixation in a meromictic lake, Lake Cadagno (Switzerland). Environ Microbiol 11:1945–1958
Heising S, Richter L, Ludwig W, Schink B (1999) Chlorobium ferrooxidans sp nov., a phototrophic green sulfur bacterium that oxidizes ferrous iron in coculture with a “Geospirillum” sp strain. Arch Microbiol 172:116–124
Holmes DE, Finneran KT, O’Neil RA, Lovley DR (2002) Enrichment of members of the family Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl Environ Microbiol 68:2300–2306
Hugenholtz P, Tyson GW (2008) Microbiology—metagenomics. Nature 455:481–483
Kalinowski BE, Johnsson A, Arlinger J, Pedersen K, Odeggrd-Jensen A, Edberg F (2006) Microbial mobilization of uranium from shale mine waste. Geomicrobiol J 23:157–164
Maidak BL, Cole JR, Lilburn TG, Parker CT, Saxman PR et al (2001) The RDP-II (Ribosomal Database Project). Nucleic Acids Res 29:173–174
Mueller-Spitz SR, Goetz GW, McLellan SL (2009) Temporal and spatial variability in nearshore bacterioplankton communities of Lake Michigan. FEMS Microbiol Ecol 67:511–522
Newton RJ, Stuart E, Eiler A, McMahon KD, Bertilsson S (2011) A guide to natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75:14–49
Overmann J (2006) Chapter 5.1 The family Chlorobiaceae. In: Dworkin M (ed) Procaryotes 7. Springer, New York, pp 359–378
Overmann J, Tuschak C (1997) Phylogeny and molecular fingerprinting of green sulfur bacteria. Arch Microbiol 167:302–309
Overmann J, Tuschak C, Frostl JM, Sass H (1998) The ecological niche of the consortium “Pelochromatium roseum”. Arch Microbiol 169:120–128
Ovreas L, Forney L, Daae FL, Torsvik V (1997) Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl Environ Microbiol 63:3367–3373
Pace NR, Stahl DA, Lane DJ, Olsen GJ (1986) The analysis of natural microbial-populations by ribosomal-RNA sequences. Adv Microb Ecol 9:1–55
Senko JM, Istok JD, Suflita JM, Krumholz LR (2002) In-situ evidence for uranium immobilization and remobilization. Environ Sci Technol 36:1491–1496
Straub KL, Schonhuber WA, Buchholz-Cleven BEE, Schink B (2004) Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiol J 21:371–378
Tonolla M, Peduzzi R, Hahn D (2005) Long-term population dynamics of phototrophic sulfur bacteria in the chemocline of Lake Cadagno, Switzerland. Appl Environ Microbiol 71:3544–3550
Ulrich KU, Ilton ES, Veeramani H, Sharp JO, Bernier-Latmani R et al (2009) Comparative dissolution kinetics of biogenic and chemogenic uraninite under oxidizing conditions in the presence of carbonate. Geochim Cosmochim Acta 73:6065–6083
VerBerkmoes NC, Denef VJ, Hettich RL, Banfield JF (2009) SYSTEMS BIOLOGY Functional analysis of natural microbial consortia using community proteomics. Nat Rev Microbiol 7:196–205
Vogl K, Glaeser J, Pfannes KR, Wanner G, Overmann R (2006) Chlorobium chlorochromatii sp nov., a symbiotic green sulfur bacterium isolated from the phototrophic consortium “Chlorochromatium aggregatum”. Arch Microbiol 185:363–372
Warnecke F, Amann R, Pernthaler J (2004) Actinobacterial 16S rRNA genes from freshwater habitats cluster in four distinct lineages. Environ Microbiol 6:242–253
Warren LA, Haack EA (2001) Biogeochemical controls on metal behaviour in freshwater environments. Earth Sci Rev 54:261–320
Weber KA, Achenbach LA, Coates JD (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–764
Xing P, Hahn MW, Wu QL (2009) Low taxon richness of bacterioplankton in high-altitude lakes of the Eastern Tibetan Plateau, with a predominance of Bacteroidetes and Synechococcus spp. Appl Environ Microbiol 75:7017–7025
Zwart G, Crump BC, Agterveld MPKV, Hagen F, Han SK (2002) Typical freshwater bacteria: an analysis of available 16S rRNA gene sequences from plankton of lakes and rivers. Aquat Microb Ecol 28:141–155
Acknowledgments
We thank Jörgen Ek and Karin Holm (Dep. of Applied Environmental Science, Stockholm University), and Klara Hajnal and Magnus Mörth (Dep. of Geological Sciences, Stockholm University) with the valuable help with analysis of nitrate and ammonium, anions using ion chromatography and metals by using ICP-OES and ICP-MS, respectively. Many thanks are due to Anna Hägglund for the assistance in the field and the laboratory. We also acknowledge Volker Brüchert (Dep. of Geological Sciences, Stockholm University) for commenting and improving the manuscript. A.F.A. and S.J.M.H. were supported by grants from the Swedish research council Formas. The pyrosequencing as well as many of the chemical analysis were financed by grants from Carl Tryggers research foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 148 kb)
Rights and permissions
About this article
Cite this article
Edberg, F., Andersson, A.F. & Holmström, S.J.M. Bacterial Community Composition in the Water Column of a Lake Formed by a Former Uranium Open Pit Mine. Microb Ecol 64, 870–880 (2012). https://doi.org/10.1007/s00248-012-0069-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-012-0069-z