Abstract
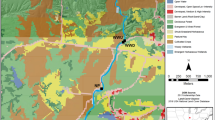
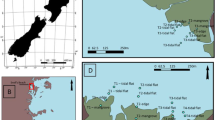
The potential effects of urbanization on the bioavailability of dissolved organic carbon (DOC) were tested by determining the extracellular enzyme activities of the heterotrophic microbial communities of the Rouge River. The activities of 19 enzymes were monitored across two water samples (river water and groundwater) at different spatial and temporal scales. High phosphatase, esterase, and aminopeptidase activities was observed in site 9 (site most exposed to anthropogenic sources) showed higher concentrations of DOC compared to sites 1 and 8 (sites exposed to less anthropogenic sources), where moderate activities of diverse range of enzymes were observed. High relative contributions of phosphatase, esterase, and aminopeptidase activities to the overall enzyme activity as observed in site 9 stressed the increased importance of peptides as C source for heterotrophic communities and high in-stream carbon processing, which account for high nonspecific extracellular enzyme activities. In contrast, high contribution of glycosyl hydrolases occurred consistently across all sites, which highlights the significance of microbial detrital and plant biomass as carbon sources. Majority of the enzymes showed evidence of activity at various extents during spring and summer. However, higher activities of leucine aminopeptidase, valine aminopeptidase, β-glucosidase, and α-mannosidase were observed in the summer; and alkaline phosphatase and α-glucosidase in the spring. The results presented here suggest a shift in organic carbon bioavailability across all sites of contrasting urbanization, despite similarities in DOC concentrations. Hence, API ZYM technique can be used as an effective indicator of river water and groundwater system health across an urban gradient.




Similar content being viewed by others
References
Ainsworth AM, Goulder R (2000) Downstream change in leucine aminopeptidase activity and leucine assimilation by epilithic microbiota along the River Swale, Northern England. Sci Total Environ 251–252:191–204
Allan JD (1995) Stream ecology: structure and function of running waters. Chapman and Hall, London, UK
Azam F (1998) Microbial control of oceanic carbon flux: The plot thickens. Science 288:694–696
Battin TJ (2000) Hydrodynamics is a major determinant of streambed biofilm activity: from the sediment to the reach scale. Limnol Oceanogr 45:1308–1319
Bidochka MJ, Burke S, Ng L (1999) Extracellular hydrolytic enzymes in fungal genus Verticulum: adaptations for pathogenesis. Can J Microbiol 45:856–864
Boon PI (1991) Enzyme activities in billabongs of southeastern Australia. In: Chorst RJ (ed) Microbial enzymes in aquatic environments. Springer, New York, pp 286–297
Boschker HTS, Cappenberg TE (1994) A sensitive method using 4-methylumbelliferyl-β- cellobiose as a substrate to measure (1,4)-β-glucanase activity in sediments. Appl Environ Microbiol 60:3592–3596
Castro NM, Hornberger GM (1991) Surface–subsurface water interactions in an alluvial mountain stream channel. Water Resour Res 27:1613–1621
Chapelle FH (2000) Ground-water microbiology and geochemistry. John Wiley and Sons, New York, p 468
Chappell KR, Goulder R (1995) A between-river comparison of extracellular-enzyme activity. Microb Ecol 29:1–17
Chrost RJ (1991) Environmental control of the synthesis and activity of aquatic microbial ectoenzymes. In: Chrost RJ (ed) Microbial enzymes in aquatic environments. Springer, New York, pp 42–50
Eriksson KE, Blanchette RA, Ander P (1990) Microbial and enzymatic degradation of wood and wood components. Springer Series in Wood Science. Springer–Verlag, Berlin
Fiebig DM, Lock MA (1991) Immobilization of dissolved organic matter from groundwater discharging through the stream bed. Freshwater Biol 26:45–55
Findlay S, Hickey CW, Quinn JM (1997) Microbial enzymatic response to catchment-scale variations in supply of dissolved organic carbon. New Zealand J Mar Freshwater Res 31:701–706
Ford TE, Naiman RJ (1989) Groundwater–surface water relationship in boreal forest watersheds: organic carbon and nutrient dynamics. Can J Fish Aquat Sci 46:41–49
Garcia-Martos P, Martin P, Hernandez-Molina JM, Garcia-Agudo L, Aoufi S, Mira J (2000) Extracellular enzymatic activity in 11 Cryptococcus species. Mycopathologia 150:1–4
Ghiorse WC, Wilson JT (1988) Microbial ecology of the terrestrial subsurface. Adv Appl Microbiol 33:107–177
Hayashi M, Rosenberry DO (2002) Effects of groundwater exchange on hydrology and ecology of surface water. Groundwater 40:309–316
Hazen TC, Jimenez L, Lopez de Victoria G, Fliermans CB (1991) Comparison of bacteria from deep subsurface sediment and adjacent groundwater. Microb Ecol 22:293–304
Hobbie JE, Daley RJ, Jasper S (1977) Use of nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33:1225–1228
Hoppe HG, Arnosti C, Herndel GF (2002) Ecological significance of bacterial enzymes in marine environment. In: Dick RP, Burns RG (eds) Enzymes in environment: Activity, ecology, and applications. CRC Press, Boca Raton, Florida, pp 73–107
Jones SE, Lock MA (1989) Hydrolytic extracellular enzyme activity in heterotrophic biofilms from two contrasting streams. Freshwater Biol 22:289–296
Kannan K, Kober JL, Kang YS, Masunaga S, Nakanishi J (2001) Polychlorinated naphthalenes, biphenyls, dibrnzo-p-dioxins, and dibenzofurans as well as polycyclic aromatic hydrocarbons and alkylphenols in sediments from the Detroit and Rouge Rivers, Michigan. USA Environ Toxicol Chem 20:1878–1889
Karrash B, Bormki K, Herzsprung G, Winkler P, Baborowski M (2003) Extracellular enzyme activity in the River Elbe during a spring flood event. Acta Hydrochim Hydrobiol 31:307–318
Keith SC, Arnosti C (2001) Extracellular enzyme activity in a river-bay-shelf transect: variations in polysaccharide hydrolysis ratio with substrate and size class. Aqua Microb Ecol 24:243–253
Khan ZU, Chugh TD, Chandy R, Provost F, Boiron P (1999) A study of the enzymatic profile of soil isolates of Nocardia asteroies. Mycopathologia 143:151–154
Kolehmainen RE, Korpela JP, Münster U, Puhakka JA, Tuovinen OH (2009) Extracellular enzyme activities and nutrient availability during artificial groundwater recharge. Water Res 43:405–416
Lebart L, Morineau A, Warwick KM (1984) Multivariate descriptive statistical analysis. John Wiley and Sons, New York
Leps J, Smilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge UniversityPress, Cambridge, UK
Madsen EL, Ghiorse WC (1993) Groundwater microbiology: subsurface ecosystem processes. In: Ford TE (ed) Aquatic microbiology: An ecological approach. Blackwell, Boston, pp 167–215
Mudryk Z, Donderski W (1997) The occurrence of heterotrophic bacteria decomposing some macromolecular components in shallow estuarine lakes. Hydrobiologia 342(243):71–78
Munster U, Chrost R (1990) Organic composition and microbial utilization of dissolved organic matter. In: Overbeck J, Chrost R (eds) Aquatic microbial ecology: Biochemical and molecular approaches. Springer, Berlin, pp 8–46
Murray KS, Farksa A, Brennan M, Czach M, Mayfield M (1997) Analysis of surface water quality in an urban watershed: Rouge River, Southeastern Michigan. Michigan Academian 24:159–171
Murray KS, Fisher LE, Therrien J, George B, Gillespie J (2001) Assessment and use of indicator bacteria to determine sources of pollution to an urban river. J Great Lakes Res 27:220–229
Palmisano AC, Scwab BS, Marusak DA (1993) Hydrolytic enzyme activity in landfilled refuse. Appl Microbiol Biotechnol 38:828–832
Palmisano AC, Marusak DA, Ritchie CJ, Schwab BS, Harper SR, Rapaport RA (1993) A novel bioreactor simulating composting of municipal solid waste. J Microbiol Method 18:99–112
Peres-Neto PR, Jackson DA, Somers KM (2003) Giving meaningful interpretation to ordination axes: assessing loading significance in principal component analysis. Ecology 84:2347–2363
Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Romani AM, Butturini A, Sabater F, Sabater S (1998) Heterotrophic metabolism in a forest stream sediment: surface versus subsurface zones. Aquat Microb Ecol 16:143–151
Shiah FK, Chen TY, Gong C, Chen C, CIang KP, Hung JI (2001) Differential coupling of bacterial and primary production in mesotrophic and oligotrophic systems of the east China Sea. Aquat Microb Ecol 23:273–282
Thorp JH, Delong MD (2002) Dominance of autochthonous autotrophic carbon in food webs of heterotrophic rivers. Oikos 96:543–550
Tiquia SM (2002) Evolution of enzyme activities during manure composting. J Appl Microbiol 92:764–775
Tiquia SM (2010) Metabolic diversity of the heterotrophic microorganisms and potential link to pollution of the Rouge River. Environ Pollut 158:1435–1443
Tiquia SM (2010) Salt-adapted bacteria isolated from the Rouge River and potential for degradation of contaminants and biotechnological applications. Environ Technol 31:967–978
Tiquia SM, Wan JHC, Tam NFY (2001) Extracellular enzyme profiles during co-composting of poultry manure and yard trimmings. Process Biochem 36:813–820
Tiquia SM, Davis D, Hadid H, Kasparian S, Ismail M, Sahly R, Shim J, Singh S, Murray KS (2007) Halophilic and halotolerant bacteria from river waters and shallow groundwater along the Rouge River of Southeastern Michigan. Environ Technol 28:297–30
Tiquia SM, Schleibak M, Schlaff J, Floyd C, Benipal B, Zakhem E, Murray KS (2008) Microbial community profiling and characterization of some heterotrophic bacterial isolates from river waters and shallow groundwater wells along the Rouge River Southeast Michigan. Environ Technol 29:651–663
Wilczek S, Fischer H, Pusch M (2005) Regulation and seasonal dynamics of extracellular enzyme activities in sediments of a large lowland river. Microbial Ecol 50:253–267
Zanardini E, Valle A, Gigliotti CJ, Papagno G, Ranalli G, Sorlini C (2002) Laboratory-scale trials of electrolytic treatment on industrial wastewaters: microbiological aspects. J Environ Sci Health A A37:1463–1481
Acknowledgments
The author thanks Hiba Hadid and Christina Floyd for the field assistance. This study was funded by the Research and Sponsored Program, University of Michigan-Dearborn.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiquia, S.M. Extracellular Hydrolytic Enzyme Activities of the Heterotrophic Microbial Communities of the Rouge River: An Approach to Evaluate Ecosystem Response to Urbanization. Microb Ecol 62, 679–689 (2011). https://doi.org/10.1007/s00248-011-9871-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9871-2