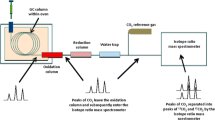
Abstract
Phospholipid fatty acid (PLFA) analysis is a robust method for characterizing soil microbial communities. We determined the effects of extraction solvent (chloroform vs dichloromethane) and buffer (phosphate vs citrate) on the yield and the profile of PLFAs extracted from an acidic (pH 5.5) and an alkaline (pH 8.6) soil following two 2-h sequential extractions. The yield and the profile of the PLFAs obtained separately by the first and the second 2-h extraction were compared to identify the impact of sequential extractions on the PLFA extraction efficiency. Chloroform–citrate and a 2 × 2-h extraction maximized PLFA yields in both soils. Multivariate analysis of the data showed that the choice of the extraction mixture did not significantly influence the profile of the PLFAs obtained by the first 2-h extraction, whereas it had a profound effect on the profile of the PLFAs obtained by the second 2-h extraction. Most PLFAs were extracted during the first extraction except 18:2ω6,9 and 22:0 which were almost equally extracted by the two sequential extractions. The choice of organic solvent significantly influenced the profile of the PLFAs extracted; their yield increased with chloroform with the exception of 18:2ω6,9 and 22:0 which were favored by dichloromethane. Overall, a 2 × 2-h extraction with chloroform/methanol/citrate is expected to provide maximum PLFA yields.



Similar content being viewed by others
Abbreviations
- PLFAs:
-
Phospholipid fatty acids
- CHL:
-
Choroform
- DCM:
-
Dichloromethane
- Cit:
-
Citrate
- Phos:
-
Phosphate
- PCA:
-
Principal component analysis
- CVA:
-
Canonical variate analysis
- CV:
-
Canonical variate
References
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bossio DA, Scow KM, Gunapala N, Graham KJ (1998) Determinants of soil microbial communities: effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microb Ecol 36:1–12
Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM (2003) A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microbiol 69:3593–3599
Cequier-Sanchez E, Rodríguez C, Ravelo AG, Arate RZ (2008) Dichloromethane as a solvent for lipid extraction and assessment of lipid classes and fatty acids from samples of different natures. J Agric Food Chem 56:4297–4303
Chaves AL, Vergara CE, Mayer JE (1995) Dichloromethane as an economic alternative to chloroform in the extraction of DNA from plant tissues. Plant Mol Biol Rep 13:18–25
Federle TW (1986) Microbial distribution in soil—new techniques. In: Megusar F, Gantar M. (eds) Perspectives in microbial ecology. Proceedings of the Fourth International Symposium on Microbial Ecology, Ljubljana, Slovenia. Slovene Society for Microbiology, pp. 493–498
Findlay RH, King GM, Watling L (1989) Efficacy of phospholipid analysis in determining microbial biomass in sediments. Appl Environ Microbiol 54:2888–2893
Findlay RH (2004) Determination of microbial community structure using phospholipids fatty acid profiles. Molecular microbial ecology manual, second edition 4.08. Kluwer, Dordrecht, pp 983–1004
Frostegård Ǻ, Tunlid A, Bååth E (1991) Microbial biomass measured as total lipid phosphate in soils of different organic content. J Microbiol Meth 14:151–163
Frostegård Ǻ, Bååth E, Tunlid A (1993) Shifts in the structure of soil microbial communities in the limed forests as revealed by phospholipid fatty acid analysis. Soil Biol Biochem 25:723–730
Fuhrmann A, Gerzabek MH, Watzinger A (2009) Effects of different chloroform stabilizers on the extraction efficiencies of phospholipid fatty acids from soils. Soil Biol Biochem 41:428–430
Gómez-Brandón M, Lores M, Domínguez J (2010) A new combination of extraction and derativization methods that reduces the complexity and preparation time in determining phospholipids fatty acids in solid environmental samples. Bioresour Technol 101:1348–1354
Hamer U, Unger M, Makeschin F (2007) Impact of air-drying and rewetting on PLFA profiles of soil microbial communities. J Plant Nutr Soil Sci 170:259–264
Hinojosa MB, Carreira JA, García-Ruíz R, Dick RP (2005) Microbial response to heavy metal-polluted soils: community analysis from phospholipids-linked fatty acids and ester-linked fatty acids extracts. J Environ Qual 34:1789–1800
Ibekwe AM, Kennedy AC (1998) Phospholipid fatty acid profiles and carbon utilization patterns for analysis of microbial community structure under field and greenhouse conditions. FEMS Microbiol Ecol 26:151–163
Lee YB, Lorenz N, Dick LK, Dick RP (2007) Cost storage and pretreatment incubation effects on soil microbial properties. Soil Sci Soc Am J 71:1299–1305
McCaig AE, Glover LA, Prosser JI (2001) Numerical analysis of grassland bacterial community structure under different land management regimens by using 16S ribosomal DNA sequence data and denaturating gradient gel electrophoresis banding patterns. Appl Environ Microbiol 67:4554–4559
Moore-Kucera J, Dick RP (2008) PLFA profiling of microbial community structure and seasonal shifts in soils of a Douglas-fir chronosequence. Microb Ecol 55:500–511
Nielsen P, Petersen SO (2000) Ester-linked polar lipid fatty acid profiles of soil microbial communities: a comparison of extraction methods and evaluation of interference from humic acids. Soil Biol Biochem 32:1241–1249
Petersen SO, Klug MJ (1994) Effects of sieving, storage, and incubation temperature on the phospholipid fatty acid profile of a soil microbial community. Appl Environ Microbiol 60:2421–2430
Piotrowska-Seget Z, Mrozik A (2003) Signature lipid biomarker (SLB) analysis in determining changes in community structure of soil microorganisms. Pol J Environ Stud 12:669–675
Rousk J, Brookes PC, Bååth E (2010) The microbial PLFA composition as affected by pH in an arable soil. Soil Biol Biochem 42:516–520
Sheldrick BH, Wang C (1993) Particle size distribution. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis, Boca Raton, pp 477–512
Snyder LR (1974) Classification of the solvent properties of common liquids. J Chromatogr 92:223–230
Spyrou IM, Karpouzas DG, Menkissoglu-Spiroudi U (2009) Do botanical pesticides alter the structure of the soil microbial community? Microb Ecol 58:715–727
O’Neil MJ, Smith A, Heckelman PE, Budavari S (2001) The Merck index—an encyclopedia of chemicals, drugs, and biologicals, 13th edn. Merck Research Laboratories, Merck & Co., Inc., Whitehouse Station, pp 369–1082
Vestal JR, White DC (1989) Lipid analysis in microbial ecology. Bioscience 39:535–541
Walkley A, Black CA (1934) An examination of the Djetgarref method for determining organic matter and proposed modification of the chromic acid titration method. Soil Sci 5:401–407
White DC, Davis WM, Nickels JS, King JD, Bobbie RJ (1979) Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 40:51–62
White DC, Pinkart HC, Ringelberg DB (1997) Biomass measurements: biochemical approaches. In: Hurst CJ, Knudsen GR, McInerney MJ, Stetzenbach LD, Walter MV (eds) Manual of environmental microbiology. ASM, Washington, DC, pp 91–101
Wu Y, Ding N, Wang G, Xu J, Wu J, Brookes PC (2009) Effects of different soil weights, storage times and extraction methods on soil phospholipids fatty acid analyses. Geoderma 150:171–178
Acknowledgments
The authors would like to thank Dr. Ioannis O. Giannakou and Dr. Theodora Matsi for providing the soil samples for this study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Papadopoulou, E.S., Karpouzas, D.G. & Menkissoglu-Spiroudi, U. Extraction Parameters Significantly Influence the Quantity and the Profile of PLFAs Extracted from Soils. Microb Ecol 62, 704–714 (2011). https://doi.org/10.1007/s00248-011-9863-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9863-2