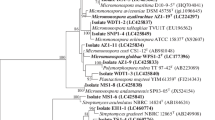
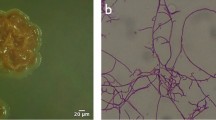
Abstract
Endophytic actinomycetes from Azadirachta indica A. Juss. were screened and evaluated for their anti-microbial activity against an array of pathogenic fungi and bacteria. A total of 55 separate isolates were obtained from 20 plants, and 60% of these showed inhibitory activity against one or more pathogenic fungi and bacteria. Actinomycetes were most commonly recovered from roots (54.5% of all isolates), followed by stems (23.6%), and leaves (21.8%). The dominant genus was Streptomyces (49.09% of all isolates), while Streptosporangium (14.5%), Microbispora (10.9%), Streptoverticillium (5.5%), Sacchromonospora sp. (5.5%), and Nocardia (3.6%) were also recovered. Streptomyces isolates AzR 006, 011, and 031 (all from roots) had acute activity against Pseudomonas fluorescens, while AzR027, 032, and 051 (also all from roots) showed activity against Escherichia coli. Meanwhile, an isolate of Nocardia sp. from leaves (AzL025) showed antagonism against Bacillus subtilis. Overall, 32 of the 55 were found to have broad spectrum significant antimicrobial activity, while about 4% of them showed strong and acute inhibition to pathogenic fungi and bacteria. Isolates of Streptomyces AzR031, 008, and 047, Nocardia sp. AzL025, and Streptosporangium sp. AzR 021 and 048 are of particular interest because they showed significant antagonistic activity against root pathogens, including Pythium and Phytophthora sp. Thus, many of the isolates recovered from A. indica in this study may be used in developing potential bio-control agents against a range of pathogenic fungi and bacteria and in the production of novel natural antimicrobial compounds. These results not only further our understanding of plant–microbe interactions but also indicate that there is an untapped resource of endophytic microorganisms that could be exploited in the biotechnological, medicinal, and agricultural industries.
Similar content being viewed by others
Abbreviations
- Az:
-
Azadirachta indica, AzS, AzL
- AzR:
-
isolate assignment code from stem, leaf, and root tissues respectively
References
Araujo JM, Silva AC, Azevedo JL (2000) Isolation of endophytic actinomycetes from root and leaves of maize (Zea mays L.). Braz Arch Biol Tech 43:447–451
Baker D (1990) Method for the isolation, culture and characterization of the Frankiaceae: soil actinomycetes and symbionts of actinorrhizal plants. In: Labeda DP (ed) Isolation of biotechnological organisms from nature. McGraw-Hill, New York, pp 213–236
Bauer AW, Kirby WM, Sherries JC, Turck M (1966) Antibiotics susceptibility testing by the standardized single disc method. Am J Clin Pathol 45:493–496
Benson DR, Silvester WB (1993) Biology of Frankia strain, actinomycetes symbionts of actinorrhizal plants. Microbiol Rev 57:293–319
Berdi J (1989) The discovery of new bioactive metabolites; screening and identification. Prog Ind Microbiol 27:3–25
Bradbury JF (1986) Guide to phytopathogenic bacteria. CAB International Mycological Institute, Kew, pp 190–197
Castillo UF, Browne L, Strobel G, Hess WM, Ezra S, Pacheco G, Ezra D (2007) Biologically active endophytic Streptomycetes from Nothofagus spp. and other plants in Patagonia. Microb Ecol 53:12–19
Cao L, Qiu Z, You J, Tan H, Zhou S (2003) Isolation and characterization of endophytic Streptomyces strains from surface sterilized tomato (Lycopersicon esculentum) roots. Lett Appl Microbiol 39:425–430
Cao LX, Qiu ZQ, You JL, Tan HM, Zhou S (2004) Isolation and characterization of endophytic Streptomyces antagonists of Fusarium wilt pathogen from surface sterilized banana roots. FEMS Microbiol Lett 247:147–152
Carroll GC (1988) Fungal endophytes in stem and leaves from latent pathogen to mutualistic symbionts. Ecology 63:2–9
Castillo UF, Strobel GA, Ford EJ, Hess WM, Porter H, Jensen JB, Albert H, Robison R (2002) Munumbicins wide spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscans. Microbiology 148:2675–2685
Coombs JT (2002) Antibiotics for wheat, Science Now! The national science forum, 20–22 August, 2002. http://www.abc.net.au/Science/news/stories/s351442.htm
Coombs JT, Franco CMM (2003) Isolation and identification of actinobacteria from surface sterilized wheat roots. Appl Env Microbiol 69:5603–5608
Crawford DL, Lynch JM, Whipps JM, Ousley MA (1993) Isolation and characterization of actinomycete antagonists of a root pathogen. Appl Env Microbiol 59:3889–3905
Doumbou CL, Akimov V, Beaulieu C (1998) Selection and characterization of microorganisms utilizing Thaxtomin A, a phytotoxin produced by Streptomyces scabies. Appl Env Microbiol 64:4313–4316
Ezra D, Castillo UF, Strobel GA, Hess WM, Porter H, Jensen JB, Condron MAM, Teplow DB, Sears J, Maranta M, Hunter M, Weber B, Yaver D (2004) Coronamycins, peptide antibiotics produced by a verticillate Streptomyces sp. (MSU-2110) endophytic on Monstera sp. Microbiology 150:785–793
Faeth SH, Hammon KE (1997) Fungal endophytes in oak tree; long term pattern of abundance and association with leaf miners. Ecology 78:810–819
Franco CMM, Cautinho LEL (1991) Detection of novel secondary metabolites. Crit Rev Biotechnol 11:193–276
Gond SK, Verma VC, Kumar A, Kumar V, Kharwar RN (2007) Study of endophytic fungal community from different parts of Aegle marmelos Correae (Rutaceae) from varanasi (India). World J Microbiol Biotechnol 23:1371–1375
Graner G, Persson P, Meijer J, Alstrom S (2003) A study on microbial diversity in different cultivars of Brassica napus in relation to its wilt pathogen, Verticillium longisporum. FEMS Microbiol Lett 224:269–276
Gupta R, Saxena RK, Chaturverde P, Virdi JS (1995) Chitinase production by Streptomyces viridificans: it’s potential in cell wall lysis. J Appl Bacteriol 78:378–383
Hawksworth DL (1991) The fungal dimension of biodiversity: magnitude, significance and conservation. Mycol Res 95:641–655
Hawksworth DL, Rossman AY (1997) Where are all the undiscribed fungi. Phytopathology 87:888–891
Katznelson H, Cole SE (1964) Production of gibberellin-like substances by bacteria and actinomycetes. Can J Microbiol 11:733–741
Kolomiets EI, Zdor NA, Romanovskaya TV, Lobanok AG (1997) Certain aspects of the phytoprotective activity of Streptomyces flavescens an antagonist of phytopathogenic fungi. Appl Biochem Microbiol 33:451–454
Kongsaeree P, Prabpai S, Sriubolmas N, Vongvein C, Wiyakrutta S (2003) Antimalarial dihydroisocoumrins produced by Geotrichum sp., an endophytic fungus of Crassocephalum crepidioides. J Nat Prod 66:709–711
Kunoh H (2002) Endophytic actinomycetes: attractive bio-control agent. J Gen Plant Pathol 68:249–252
Mahesh B, Tejesvi MV, Nalini MS, Prakash HS, Kini KR, Subbiah V, Hunthrike SS (2005) Endophytic mycoflora of inner bark of Azadirachta indica A. Juss. Curr Sci 88:218–219
Matsumoto A, Takahashi Y, Mochizuki M, Seino A, Iwai Y, Omura S (1998) Characterization of actinomycetes isolated from fallen leaves. Actinomycetologica 12:46–48
Pullen C, Schmitz P, Meurer K, Bamberg DD, Lohman S, Franca SDC, Groth I, Schlegel B et al (2002) New and bioactive compounds from Streptomyces strains residing in the wood of celasatraceae. Planta 216:162–167
Rajagopal R, Suryanarayanan TS (2000) Isolation of endophytic fungi from leaves of neem (Azadirachta indica). Curr Sci 78:1375–1378
Ruan JS, Liu ZH, Liang LN, Yang DC (1990) Study and application of actinomycetes. Bios-Scientific, Beijing, pp 1–18 ISBN 7-03-001499-5
Sardi P, Saracchi M, Quaroni S, Petrolini B, Borgonovi GE, Merli S (1992) Isolation of endophytic Streptomyces strains from surface sterilized roots. Appl Env Microbiol 58:2691–263
Schulz B, Wanke U, Draeger S, Aust HJ (1993) Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol Res 97:1447–1450
Sessitch A, Reiter B, Pfeifer U, Wilhelm E (2001) Cultivation independent population analysis of bacterial endophytes in three potato varieties based on eubacterial and actinomycetes specific PCR of 16S r RNA genes. FEMS Microbiol Ecol 1305:1–10
Strobel GA, Long DM (1998) Endophytic microbes embody pharmaceutical potential. ASM News 64:263–268
Strobel GA, Yang XS, Sears J, Kramer R, Sidhu RS, Hess WM (1996) Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallichiana. Microbiology 142:435–440
Tan RX, Zou WX (2001) Endophytes: a rich source of functional metabolites. Nat Prod Rep 18:448–459
Valois D, Fayad K, Barasubiye T, Garon M, dery C, Brzezinski R, Beaulieu C (1996) Glucanolytic actinomycetes antagonistic to Phytophthora fragariae var. rubi, the causal agent of raspberry root rot. Appl Env Microbiol 62:1630–1635
Verma VC, Gond SK, Kumar A, Kharwar RN, Strobel GA (2007) Endophytic mycoflora from leaf stem and bark tissues of Azadirachta indica A Juss (Neem) from Varanasi (India). Microb Ecol 54:119–125
Yan XC (1992) Classification and identification of actinomycetes. Bios Scientific, Beijing, pp 623–656 ISBN 7-03-002022-7
Yuan WM, Crawford DL (1995) Characterization of Streptomyces lydicus WYEC108 as a potential agent against fungal root and seed rot. Appl Env Microbiol 61:3119–3128
Acknowledgments
Authors (VCV, SKG, and AK) are grateful to CSIR and UGC for funding their fellowships in the form of JRF and SRF. We thank to the Head and Coordinator, CAS in Botany, BHU, Varanasi for providing the necessary facilities available in the department. Authors woe their sincere thanks to Dr. Ragini Tilak, (IMS, BHU) for providing human bacterial and fungal pathogens.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, V.C., Gond, S.K., Kumar, A. et al. Endophytic Actinomycetes from Azadirachta indica A. Juss.: Isolation, Diversity, and Anti-microbial Activity. Microb Ecol 57, 749–756 (2009). https://doi.org/10.1007/s00248-008-9450-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-008-9450-3