Abstract
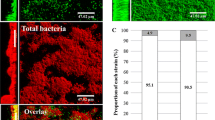
Mass mortalities of larval cultures of Chilean scallop Argopecten purpuratus have repeatedly occurred in northern Chile, characterized by larval agglutination and accumulation in the bottom of rearing tanks. The exopolysaccharide slime (EPS) producing CAM2 strain was isolated as the primary organism from moribund larvae in a pathogenic outbreak occurring in a commercial hatchery producing larvae of the Chilean scallop Argopecten purpuratus located in Bahía Inglesa, Chile. The CAM2 strain was characterized biochemically and was identified by polymerase chain reaction amplification of 16S rRNA as Halomonas sp. (Accession number DQ885389.1). Healthy 7-day-old scallop larvae cultures were experimentally infected for a 48-h period with an overnight culture of the CAM2 strain at a final concentration of ca. 105 cells per milliliter, and the mortality and vital condition of larvae were determined by optical and scanning electron microscopy (SEM) to describe the chronology of the disease. Pathogenic action of the CAM2 strain was clearly evidenced by SEM analysis, showing a high ability to adhere and detach larvae velum cells by using its “slimy” EPS, producing agglutination, loss of motility, and a posterior sinking of scallop larvae. After 48 h, a dense bacterial slime on the shell surface was observed, producing high percentages of larval agglutination (63.28 ± 7.87%) and mortality (45.03 ± 4.32%) that were significantly (P < 0.05) higher than those of the unchallenged control cultures, which exhibited only 3.20 ± 1.40% dead larvae and no larval agglutination. Furthermore, the CAM2 strain exhibited a high ability to adhere to fiberglass pieces of tanks used for scallop larvae rearing (1.64 × 105 cells adhered per square millimeters at 24 h postinoculation), making it very difficult to eradicate it from the culture systems. This is the first report of a pathogenic activity on scallop larvae of Halomonas species, and it prompts the necessity of an appraisal on biofilm-producing bacteria in Chilean scallop hatcheries.







Similar content being viewed by others
References
Anderson C, Atlar M, Callow M, Candries M, Townsin RL (2003) The development of foul-release coatings for seagoing vessels. J Mar Des Operat B4:11–23
Amaro AM, Fuentes MS, Ogalde SR, Venegas JA, Suárez-Isla BA (2005) Identification and characterization of potentially algal-lytic marine bacteria strongly associated with the toxic dinoflagellate Alexandrium catenella. J Eukaryot Microbiol 52:191–200
Arahal DR, Ventosa A (2006) The Family Halomonadaceae. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes. A handbook on the biology of bacteria, vol. 6. Proteobacteria: gamma subclass. 3rd edn. Springer, New York, pp 811–835
Arahal DR, Ludwig W, Schleifer K-H, Ventosa A (2002) Phylogeny of the family Halomonadaceae based on 23S and 16S rDNA sequence analyses. Int J Syst Evol Microbiol 52:241–249
Armstrong E, Boyd KG, Burgess JG (2000) Prevention of marine biofouling using natural compounds from marine organisms. Biotechnol Annu Rev 6:221–241
Avendaño RE, Riquelme CE, Escribano R, Reyes N (2001) Sobrevivencia y crecimiento de post-larvas de Argopecten purpuratus (Lamarck, 1819) em Bahia Inglesa, Chile: efectos del origen, distribución en la bahía y bacterioflora larval. Rev Chil Hist Nat 74:669–679
Barrow GI, Feltham RKA (1993) Cowan and Steel’s manual for the identification of medical bacteria, 3rd edn. Cambridge University Press, Cambridge 331 p
Bassler BL (1999) How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol 2:582–587
Bouchotroch S, Quesada E, Izquierdo I, Rodríguez M, Béjar V (2000) Bacterial exopolysaccharides produced by newly discovered bacteria belonging to the genus Halomonas, isolated from hypersaline habitats in Morocco. J Ind Microbiol Biotech 24:374–378
Clarkson N (2000) The antifouling potential of silicone elastomer polymers. In: Fingermann M, Nagabhushanam R, Thompson MF (eds) Recent advances in marine biotechnology, vol 3. Biofilms, bioadhesion, corrosion and biofouling. Science Publishers, USA, pp 147–171
de Nys R, Steinberg PD (2002) Linking marine biology and biotechnology. Curr Opin Biotechnol 13:244–248
Dobretsov S, Dahms HU, YiLi H, Wahl M, Qian PY (2007) The effect of quorum sensing blockers on the formation of marine microbial communities and larval attachment. FEMS Microbiol Ecol 60:177–188
Elston RA (1984) Prevention and management of infectious diseases in intensive mollusc husbandry. J World Maric Soc 15:284–300
Farias A, Uriarte I, Castilla JC (1998) A biochemical study of the larval and postlarval stages of the Chilean scallop Argopecten purpuratus. Aquaculture 166:37–47
Gherna LR (1994) Culture preservation. In: Gerhardt P, Murray REG, Wood WA, Krieg NR (eds) Methods for general and molecular bacteriology. American Society for Microbiology, Washington, DC, pp 278–292
Gómez-León J, Villamil L, Lemos ML, Novoa B, Figueras A (2005) Isolation of Vibrio alginolyticus and Vibrio splendidus from Aquacultured Carpet Shell Clam (Ruditapes decussatus) Larvae Associated with Mass Mortalities. Appl Environ Microbiol 71:98–104
Hansen GH, Sörheim R (1991) Improved method for phenotypical characterization of marine bacteria. J Microbiol Methods 13:231–241
Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Hoiby N, Givskov M (2003) Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 22:3803–3815
Jeffries V (1982) Three Vibrio strains pathogenic to larvae of Crassostrea virginica and Ostrea edulis. Aquaculture 29:201–206
Kjelleberg S, Steinberg P, Givskov M, Gram L, Manefield M, de Nys R (1997) Do marine natural products interfere with prokaryotic AHL regulatory systems. Aquat Microb Ecol 13:85–93
Lee OO, Qian P (2003) Chemical control of bacterial epibiosis and larval settlement of Hydroides elegans in red sponge Myscale adherens. Biofouling 19:171–180
Lodeiros C, Bolinches J, Dopazo C, Toranzo A (1987) Bacillary necrosis in hatcheries of Ostrea edulis in Spain. Aquaculture 65:15–29
Mah T-FC, O’Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39
Maki JS, Ding L, Stokes J, Kavouras JH, Rittschof D (2000) Substratum/bacterial interactions and larval attachment: films and exopolysaccharides of Halomonas marina (ATCC 25374) and their effect on barnacle cyprid larvae, Balanus amphitrite Darwin. Biofouling 16:159–170
Mata JA, Martínez-Canovas J, Quesada E, Béjar V (2002) A detailed phenotypic characterisation of the type strains of Halomonas species. Syst Appl Microbiol 25:360–375
Miranda CD, Rojas R (2006) Copper accumulation by bacteria and transfer to scallop larvae. Mar Pollut Bull 52:293–300
Nevell TG, Edwards DP, Davies AJ, Pullin RA (1996) The surface properties of silicone elastomers exposed to seawater. Biofouling 10:199–212
Nicolas JL, Corre S, Gauthier G, Robert R, Ansquer D (1996) Bacterial problems associated with scallop Pecten maximus larval culture. Dis Aquat Org 27:67–76
Norusis MJ (2004) Statistical Package for the Social Sciences (SPSS) 12.0 Statistical Procedures Companion. Prentice Hall, Englewood Cliffs, USA 601 p
Nottage A, Birkbeck TH (1986) Toxicity of marine bivalves of culture supernatant fluids of the bivalve-pathogenic Vibrio strain NCMB 1338 and other vibrios. J Fish Dis 9:249–256
Nottage A, Birkbeck TH (1987) The role of toxins in Vibrio infections of bivalve molluscs. Aquaculture 67:244–246
Olson ME, Ceri H, Morck DW, Buret AG, Read RR (2002) Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res 66:86–92
Prado S, Romalde JL, Montes J, Barja JL (2005) Pathogenic bacteria isolated from disease outbreaks in shellfish hatcheries. First description of Vibrio neptunius as an oyster pathogen. Dis Aquat Org 67:209–215
Prieur D, Nicolas JL, Plusquellec A, Vigneulle M (1990) Interaction between bivalve molluscs and bacteria in the marine environment. Oceanogr Mar Biol Annu Rev 28:277–352
Riquelme C, Chavez P, Morales Y, Hayashida G (1994) Evidence for parental bacterial transfer to larvae in Argopecten purpuratus (Lamarck, 1819). Biol Res 27:129–134
Riquelme C, Hayashida G, Toranzo A, Vilches J, Chavez P (1995) Pathogenicity studies on a Vibrio anguillarum-related (VAR) strain causing an epizootic in Argopecten purpuratus larvae cultured in Chile. Dis Aquat Org 22:135–141
Riquelme C, Hayashida G, Vergara N, Vasquez A, Morales Y, Chavez P (1995) Bacteriology of the scallop Argopecten purpuratus (Lamarck, 1819) cultured in Chile. Aquaculture 138:49–60
Riquelme C, Toranzo A, Barja J, Vergara N, Araya R (1996) Association of Aeromonas hydrophila and Vibrio alginolyticus with larval mortalities of scallop (Argopecten purpuratus). J Invert Pathol 67:213–218
Robert R, Miner P, Nicolas JL (1996) Mortality control of scallop larvae in the hatchery. Aquac Int 4:305–313
Rosenblum BB, Lee LG, Spurgeon SL, Khan SH, Menchen SM, Heiner CR, Chen SM (1997) New dye-labeled terminators for improved DNA sequencing patterns. Nucleic Acids Res 25:4500–4504
Sainz J, Maeda-Martínez A, Ascencio F (1998) Experimental vibriosis induction with Vibrio alginolyticus of larval of the Caterina scallop (Argopecten ventricosis=circularis) (Sowerby II, 1842). Microb Ecol 35:188–192
Schulze AD, Alabib AO, Tattersall-Sheldrakeb AR, Miller KM (2006) Bacterial diversity in a marine hatchery: balance between pathogenic and potentially probiotic bacterial strains. Aquaculture 256:50–73
Valsecchi E (1998) Tissue boiling: a short-cut in DNA extraction for large-scale population screenings. Mol Ecol 7:1243–1245
Walne PR (1965) Observations on the influence of food supply and temperature on the feeding and growth of the larvae of Ostrea edulis L. Fish Invest II 24:1–45
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice-Hall, Inc., Upper Saddle River, NJ, USA 931 p
Acknowledgments
This study was partially supported by the Project Grant PT-10 from the Developing and Innovation Funding (FDI-CORFO) of Chile. We would like to acknowledge the cooperation of Chilean scallop hatchery managers Alejandro Abarca and Christian Tapia who, over many years, have freely collaborated with us to solve their problems. We also thank MSc. Marisol Romero from Electronic Microscopy Unit of the Universidad Católica del Norte for her expert technical assistance. The comments and suggestions of the reviewers are greatly appreciated as they helped to improve the presentation of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rojas, R., Miranda, C.D. & Amaro, A.M. Pathogenicity of a Highly Exopolysaccharide-producing Halomonas Strain Causing Epizootics in Larval Cultures of the Chilean Scallop Argopecten purpuratus (Lamarck, 1819). Microb Ecol 57, 129–139 (2009). https://doi.org/10.1007/s00248-008-9401-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-008-9401-z