Abstract
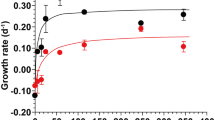
Experimental studies were carried out on an Antarctic isolate of the heterotrophic nanoflagellate Paraphysomonas imperforata to examine the efficiency of incorporation and remineralization of nitrogen and phosphorus from bacterial prey. Experiments were carried out over a temperature range from ambient Antarctic temperature (0 °C) to 10 °C. Temperature had a marked effect on the maximal growth rate of the phagotrophic nanoflagellate. Growth rate in the presence of high prey abundance ranged from 0.6 day−1 at 0 °C to 2.6 day−1 at 10 °C. In contrast, temperature had no discernable effect on the efficiencies of incorporation and remineralization of major nutrients by P. imperforata. The efficiencies of phosphorus and nitrogen incorporation from prey biomass averaged over the temperature range examined were 58 and 39%, respectively, for the two elements. Ammonium and phosphate were the dominant forms of dissolved nitrogen and phosphorus appearing in the culture medium during the grazing phase of the experiments. Overall, dissolved organic nitrogen and phosphorus constituted minor components of these elements released by the grazing activities of the protist. The results of this study indicated that incorporation/remineralization of nitrogen and phosphorus contained in prey was relatively unaffected by culture temperature in this heterotrophic nanoflagellate, although low temperature significantly depressed its growth rate. This finding has important implications for energy utilization and elemental cycling in perennially cold ecosystems and is at odds with conclusions that have been reached in some previous studies regarding the growth efficiency of phagotrophic Antarctic protists.





Similar content being viewed by others
References
Andersen OK, Goldman JC, Caron DA, Dennett MR (1986) Nutrient cycling in a microflagellate food chain: III. Phosphorus dynamics. . Mar Ecol Prog Ser 31:47–55
Caron DA (1990) Growth of two species of bacterivorous nanoflagellates in batch and continuous culture, and implications for their planktonic existence. Mar Microb Food Webs 4:143–159
Caron DA, Goldman JC (1990) Protozoan nutrient regeneration. In: Capriulo GM (ed) Ecology of marine protozoa. Oxford University Press, New York, pp 283–306
Caron DA, Goldman JC, Dennett MR (1986) Effect of temperature on growth, respiration and nutrient regeneration by an omnivorous microflagellate. Appl Environ Microbiol 52:1340–1347
Caron DA, Porter KG, Sanders RW (1990) Carbon, nitrogen and phosphorus budgets for the mixotrophic phytoflagellate Poterioochromonas malhamensis (Chrysophyceae) during bacterial ingestion. Limnol Oceanogr 35:433–443
Caron DA, Goldman JC, Andersen OK, Dennett MR (1985) Nutrient cycling in a microflagellate food chain: II. Population dynamics and carbon cycling. . Mar Ecol Prog Ser 24:243–254
Caron DA, Lim EL, Sanders RW, Dennett MR, Berninger UG (2000) Responses of bacterioplankton and phytoplankton to organic carbon and inorganic nutrient additions in contrasting oceanic ecosystems. Aquat Microb Ecol 22:175–184
Choi JW, Peters F (1992) Effects of temperature on two psychrophilic ecotypes of a heterotrophic nanoflagellate, Paraphysomonas imperforata. Appl Environ Microbiol 58:593–599
Cotner JB, Ammerman JW, Peele ER, Bentzen E (1997) Phosphorus-limited bacterioplankton growth in the Sargasso Sea. Aquat Microb Ecol 13:141–149
del Giorgio PA, Bird DF, Prairie YT, Planas D (1996) Flow cytometric determination of bacterial abundance in lake plankton with the green nucleic acid stain SYTO 13. Limnol Oceanogr 41:783–789
Dolan JR (1997) Phosphorus and ammonia excretion by planktonic protists. Mar Geol 139:109–122
Finlay BJ, Clarke KJ (1999) Apparent global ubiquity of species in the protist genus Paraphysomonas. Protist 150:419–430
Goldman JC, Caron DA, Dennett MR (1987) Nutrient cycling in a microflagellate food chain: IV. Phytoplankton-microflagellate interactions. Mar Ecol Prog Ser 38:75–87
Goldman JC, Caron DA, Andersen OK, Dennett MR (1985) Nutrient cycling in a microflagellate food chain: I. Nitrogen dynamics. Mar Ecol Prog Ser 24:231–242
Hargrave BT, Geen GH (1968) Phosphorus excretion by zooplankton. Limnol Oceanogr 13:332–342
Ikeda T, Hing Fay E, Hutchinson SA, Boto GM (1982) Ammonia and inorganic phosphate excretion by zooplankton from inshore waters of the Great Barrier Reef, Queensland. I. Relationship between excretion rates and body size. Aust J Mar Freshwater Res vol. 33. 55–70
Ishigaki T, Sleigh MA (2001) Grazing characteristics and growth efficiencies at two different temperatures for three nanoflagellates fed with Vibrio bacteria at three different concentrations. Microb Ecol 41:264–271
Laybourn J, Stewart JM (1975) Studies on consumption and growth in the ciliate Colpidium campylum Stokes. J Anim Ecol 44:165–174
Leboy PS, Cline SG, Conner RL (1964) Phosphate, purines and pyrimidines as excretory products of Tetrahymena. J Protozool 11:217–222
Lee CC, Fenchel T (1972) Studies on ciliates associated with sea ice from Antarctica. II. Temperature responses and tolerances in ciliates from Antarctic, temperate and tropical habitats. Archiv fur Protistenk 114:237–244
Mayes DF, Rogerson A, Marchant H, Laybourn-Parry J (1997) Growth and consumption rates of bacterivorous Antarctic naked marine amoebae. Mar Ecol Prog Ser 160:101–108
Menzel D, Corwin N (1965) The measurement of total phosphorus in seawater based on the liberation of organically bound fractions by persulfate oxidation. Limnol Oceanogr 10:280–282
Nagata T, Kirchman DL (1991) Release of dissolved free and combined amino-acids by bacterivorous marine flagellates. Limnol Oceanogr 36:433–443
Nagata T, Kirchman DL (1992) Release of macromolecular organic-complexes by heterotrophic marine flagellates. Mar Ecol Prog Ser 83:233–240
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon, New York
Preisig HR, Hibberd DJ (1982) Ultrastructure and taxonomy of Paraphysomonas (Chrysophyceae) and related genera I. Nord J Bot 2:397–420
Rassoulzadegan F (1982) Dependence of grazing rate, gross growth efficiency and food size range on temperature in a pelagic oligotrichous ciliate Lohmanniella spiralis Leeg., fed on naturally occurring particulate matter. Ann Inst Oceanogr 58:177–184
Rogerson A (1981) The ecological energetics of Amoeba proteus (Protozoa). Hydrobiologia 85:117–128
Rose JM, Caron DA (2007) Does low temperature constrain the growth rates of heterotrophic protists? Evidence and implications for algal blooms in cold waters. Limnol Oceanogr 52:886–895
Sherr BF, Sherr EB, Berman T (1983) Grazing, growth and ammonium excretion rates of a heterotrophic microflagellate fed with four species of bacteria. Appl Environ Microbiol 45:1196–1201
Sherr EB, Sherr BF (2002) Significance of predation by protists in aquatic microbial food webs. A Van Leeuw J Microb 81:293–308
Soldo AT, Wagtendonk WJ (1961) Nitrogen metabolism in Paramecium aurelia. J Protozool 8:41–55
Soldo AT, Godoy GA, Larin F (1978) Purine-excretory nature of refractile bodies in the marine ciliate Parauronema acutum. J Protozool 25:416–418
Stoecker DK (1984) Particle production by planktonic ciliates. Limnol Oceanogr 29:930–940
Strom SL, Miller CB, Frost BW (2000) What sets lower limits to phytoplankton stocks in high-nitrate, low-chlorophyll regions of the open ocean? Mar Ecol Prog Ser 193:19–31
Thingstad TF, Skjoldal EF, Bohne RA (1993) Phosphorus cycling and algal-bacterial competition in Sandsfjord, Western Norway. Mar Ecol Prog Ser 99:239–259
Verity PG (1985) Grazing, respiration, excretion, and growth rates of tintinnids. Limnol Oceanogr 30:1268–1282
Wilcox RR (2003) Applying contemporary statistical techniques. Academic, New York
Acknowledgments
We gratefully acknowledge M. Dennett for collection of the Antarctic water samples, D. Moran for isolation of the flagellate culture, and A. Thompson for assistance with the transmission electron microscope. Funding was provided by National Science Foundation grants OPP-0125437 and ANT-0528715.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rose, J.M., Vora, N.M. & Caron, D.A. Effect of Temperature and Prey Type on Nutrient Regeneration by an Antarctic Bacterivorous Protist. Microb Ecol 56, 101–111 (2008). https://doi.org/10.1007/s00248-007-9328-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9328-9