Abstract
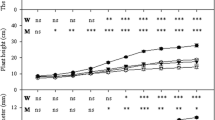
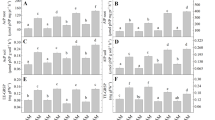
This study compared the effectiveness of four arbuscular mycorrhizal (AM) fungal isolates (two autochthonous presumably drought-tolerant Glomus sp and two allochthonous presumably drought-sensitive strains) on a drought-adapted plant (Lavandula spica) growing under drought conditions. The autochthonous AM fungal strains produced a higher lavender biomass, specially root biomass, and a more efficient N and K absorption than with the inoculation of similar allochthonous strains under drought conditions. The autochthonous strains of Glomus intraradices and Glomus mosseae increased root growth by 35% and 100%, respectively, when compared to similar allochthonous strains. These effects were concomitant with an increase in water content and a decline in antioxidant compounds: 25% glutathione, 7% ascorbate and 15% H2O2 by G. intraradices, and 108% glutathione, 26% ascorbate and 43% H2O2 by G. mosseae. Glutathione and ascorbate have an important role in plant protection and metabolic function under water deficit; the low cell accumulation of these compounds in plants colonized by autochthonous AM fungal strains is an indication of high drought tolerance. Non-significant differences between antioxidant activities such as glutathione reductase (GR), catalase (CAT) and superoxide dismutase (SOD) in colonized plants were found. Thus, these results do not allow the generalization that GR, CAT and SOD were correlated with the symbiotic efficiency of these AM fungi on lavender drought tolerance. Plants colonized by allochthonous G. mosseae (the less efficient strain under drought conditions) had less N and K content than those colonized by similar autochthonous strain. These ions play a key role in osmoregulation. The AM symbiosis by autochthonous adapted strains also produced the highest intraradical and arbuscular development and extraradical mycelial having the greatest fungal SDH and ALP-ase activities in the root systems. Inoculation of autochthonous drought tolerant fungal strains is an important strategy that assured the greatest tolerance water stress contributing to the best lavender growth under drought.







Similar content being viewed by others
References
Aebi, H (1984) Catalase in vitro. In: Packer, L (Ed.) Methods in Enzymology, vol 105. Oxygen Radicals in Biological Systems, Academic Press, London, pp 121–126
Allen, EB, Allen, MF (1986) Water relations of xeric grasses in the field: interactions of mycorrhizas and competition. New Phytol 104: 559–571
Aono, M, Kubo, A, Saji, H, Tanaka, K, Kondo, N (1993) Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplastic glutathione-reductase activity. Plant Cell Physiol 34: 129–135
Augé, RM (2001) Water relations, drought and vesicular–arbuscular mycorrhizal symbiosis. Mycorrhiza 11: 3–42
Azcón, R, Barea, JM (1997) Mycorrhizal dependency of a representative plant species in mediterranean shrublands (Lavandula spica) as a key factor to its use for revegetation strategies in desertification-threatened areas. Appl Soil Ecol 7:83–92
Barea, JM, Azcón, R, Azcón-Aguilar, C (1992) Vesicular-arbuscular mycorrhizal fungi in nitrogen-fixing systems. In: Norris, JR, Read, DJ, Varma, A (Eds.) Methods in Microbiology, vol 24. Techniques for the Study of Mycorrhizae, Academic Press, London, pp 391–416
Barea, JM, Azcón, R, Azcón-Aguilar, C (2002) Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie Van Leeuwenhoek 81: 343–351
Barea, JM, Jeffries, P (1995) Arbuscular mycorrhizas in sustainable soil plant systems. In: Varma, A, Hock, B (Eds.) Mycorrhiza: Structure, Function, Molecular Biology and Biotechnology, Springer-Verlag, Heidelberg, pp 521–559
Beyer, WF, Fridovich, I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161: 559–566
Bowler, C, Vanmontagu, M, Inze, D (1992) Superoxide-dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43: 83–116
Bremner, JM (1996) Nitrogen total. In: Sparks, DL (Ed.) Methods of Soil Analysis. Part 3: Chemical Methods, Soil Science Society of America, American Society of Agronomy, Madison, USA, pp 1085–1121
Brennan, T, Frenkel, C (1977) Involvement of hydrogen-peroxide in regulation of senescence in pear. Plant Physiol 59: 411–416
Bryla, DR, Duniway, JM (1997) Growth, phosphorus uptake, and water relations of safflower and wheat infected with an arbuscular mycorrhizal fungus. New Phytol 136: 581–590
Bryla, DR, Duniway, JM (1997) Water uptake by safflower and wheat roots infected with arbuscular mycorrhizal fungi. New Phytol 136: 591–601
Burrit, DJ, Larkindale, J, Hurd, CL (2002). Antioxidant metabolism in the intertidal red seaweed stictosiphonia arbuscular following desiccation. Planta 215: 829–838
Caravaca, F, Barea, JM, Palenzuela, J, Figueroa, D, Alguacil, MD, Roldan, A (2002) Establishment of shrubs species in a degraded semiarid site after inoculation with native or allochtohonous arbuscular mycorrhizal fungi. Appl Soil Ecol 22: 103–111
Carlberg, I, Mannervik, B (1985) Glutathione reductase. Methods Enzymol 113: 484–489
Duncan, DB (1955) Multiple range and multiple F tests. Biometrics 11: 1–42
Essington, ME (2004) Soil and Water Chemistry: an Integrative Approach. CRC Press, Boca Raton, Florida
Foyer, CH, López-Delgado, H, Dat, JF, Scott, IM (1997) Hydrogen peroxide and glutathiones-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant 100: 241–254
Foyer, CH, Souriau, N, Perret, S, Lelandais, M, Kunert, KJ, Pruvost, C, Jouanin, L (1995) Overexpression of glutathione-reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol 109: 1047–1057
Goicoechea, N, Szalai, G, Antolín, MC, Sánchez-Díaz, M, Paldi, E (1998) Influence of arbuscular mycorrhizar and Rhizobium on free polyamines and proline levels in water-stressed alfalfa. J Plant Physiol 153: 706–711
Koide, RT (1993) Physiology of the mycorrhizal plant. Adv Plant Pathol 9: 33–54
Lachica, M, Aguilar, A, Yañez, J (1973) Análisis foliar, métodos utilizados en la Estación Experimental del Zaidín. An Edafol Agrobiol 32: 1033–1047
Law, MY, Charles, SA, Halliwell, B (1992) Glutathione and ascorbic acid in spinach (Spicacea oleracea) choloplast. The effect of hydrogen peroxide and Paraquat. Biochem J 210: 899–903
Levine, A, Tenhaken, R, Dixon, R, Lamb, C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593
Marulanda, A, Barea, JM, Azcón, R (2006) An indigenous drought-tolerant strain of Glomus intraradices associated with a native bacterium improves water transport and root development in Retama sphaerocarpa. Microb Ecol 52:670–678
Marulanda, A, Azcón, R, Ruíz-Lozano, JM (2003) Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiol Plant 119: 526–533
Monzón, A, Azcón, R (1996) Relevance of mycorrhizal fungal origin and host plant genotype to inducing growth and nutrient uptake in Medicago species. Agric Ecosyst Environ 60: 9–15
Niu, DK, Wang, MG, Wang, YF (1997) Plant cellular osmotic. Acta Biotheor 45: 161–169
Noctor, G, Foyer, CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279
Phillips, JM, Hayman, DS (1970) Improved procedure of clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55: 159–161
Porcel, R, Barea, JM, Ruíz-Lozano, JM (2003) Antioxidant activities in mycorrhizal soybean under drought stress and their possible relationship to the process of nodule senescence. New Phytol 157: 135–143
Requena, N, Jeffries, P, Barea, JM (1996) Assessment of natural mycorrhizal potential in a desertified semiarid ecosystem. Appl Environ Microbiol 62: 842–847
Rontein, D, Basset, G, Hanson, AD (2002) Metabolic engineering of osmoprotectant accumulation in plants. Metab Eng 4: 49–56
Roth, CH, Malicki, MA, Plagge, R (1992) Empirical evaluation of the relationship between soil dielectric constant and volumetric water content as the basis for calibrating soil moisture measurements. J Soil Sci 43: 1–13
Ruíz-Lozano, JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13: 309–317
Ruíz-Lozano, JM, Azcón, R (1995) Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol Plant 95: 472–478
Ruíz-Lozano, JM, Azcón, R (1996) Mycorrhizal colonization and drought stress as factors affecting nitrate reductase activity in lettuce plants. Agric Ecosyst Environ 60: 175–181
Ruíz-Lozano, JM, Azcón, R, Gómez, M (1995) Effects of arbuscular-mycorrhizal Glomus species on drought tolerance: physiological and nutritional plant responses. Appl Environ Microbiol 61: 456–460
Smith, SE, Gianinazzi-Pearson, V (1990) Phosphate uptake and arbuscular activity in mycorrhizal Allium cepa L. Effects of photon irradiance and phosphate nutrition. Aust J Plant Physiol 17: 177–188
Sumner, ME, Miller, WP (2007) Cation exchange capacity and exchange coefficients. In: Sparks, DL (Ed.) Methods of Soil Analysis. Part 3: Chemical Methods, Soil Science Society of America, American Society of Agronomy, Madison, USA, pp 1201–1229
Thomas, HM, Morgan, WG, Humphreys, MW (2003) Designing grasses with a future—combining the attributes of Lolium and Festuca. Euphytica 133: 19–26
Tisserant, B, Gianinazzi-Pearson, V, Gianinazzi, S, Gollotte, A (1993) In Planta histochemical staining of fungal alkaline phosphatase activity for analysis of efficient arbuscular mycorrhizal infections. Mycol Res 97: 245–250
Trouvelot, A, Fardeau, JC, Plenchette, C, Gianinazzi, S, Gianinazzi-Pearson, V (1986) Nutritional balance and symbiotic expression in mycorrhizal wheat. Physiol Veg 24: 300
Vilariño, A, Arines, J (1990) An instrumental modification of Gerdeman and Nicolson’s method for extracting VAM fungal spores from soil samples. Plant Soil 121: 211–215
Vivas, A, Azcón, R, Biró, B, Barea, JM, Ruíz-Lozano, JM (2003) Influence of bacterial strains isolated from lead-polluted soil and their interactions with arbuscular mycorrhizae on the growth of Trifolium pratense L. under lead toxicity. Can J Microbiol 49: 577–588
White, I, Knight, J.H., Zegelin, SJ, Topp, GC (1994) Comments to “Considerations on the use of time-domain reflectometry (TDR) for measuring soil water content” by WR Whalley. J Soil Sci 45: 503–508
Wright, SF, Franckee-Snyder, M, Morton, M, Upadhyaya, A (1998) Time-course study and partial characterization of a protein on arbuscular mycorrhizal hyphae during active colonization of roots. Plant Soil 181: 193–203
Wright, SF, Nichols, KA, Schmidt, WF (2006) Comparison of efficacy of three extractants to solubilize glomalin on hyphae and soil. Chemosphere 64(7): 1219–1224
Wright, SF, Upadhyaya, A (1996) Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci 161: 1–12
Acknowledgments
This study was supported by GLO FEDER-National Plan (REN2003-00968). A. M. acknowledges AECI for financial support through a MAE Fellowship. We thank Dra. C. Lluch Plá and her group for the help in the determination of antioxidant activities, C. Cano who give inoculants of fungi AM from de E.E.Z collection and R. Aroca, R. Blanco, and F. Jahromi for correcting the English text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marulanda, A., Porcel, R., Barea, J.M. et al. Drought Tolerance and Antioxidant Activities in Lavender Plants Colonized by Native Drought-tolerant or Drought-sensitive Glomus Species. Microb Ecol 54, 543–552 (2007). https://doi.org/10.1007/s00248-007-9237-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9237-y