Abstract
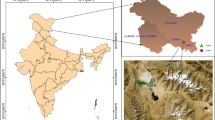
The diversity of methanogenic archaea in enrichment cultures established from the sediments of Lonar Lake (India), a soda lake having pH ≈ 10, was investigated using 16S rDNA molecular phylogenetic approach. Methanogenic enrichment cultures were developed in a medium that simulated conditions of soda lake with three different substrates viz., H2:CO2, sodium acetate, and trimethylamine (TMA), at alkaline pH. Archaeal 16S rRNA clone libraries were generated from enrichment cultures and 13 RFLP groups were obtained. Representative sequence analysis of each RFLP group indicated that the majority of the 16S rRNA gene sequences were phylogenetically affiliated with uncultured Archaea. Some of the groups may belong to new archaeal genera or families. Three RFLP groups were related to Methanoculleus sp, while two related to Methanocalculus sp. 16S rRNA gene sequences found in Lonar Lake were different from sequences reported from other soda lakes and more similar to those of oil reservoirs, palm oil waste treatment digesters, and paddy fields. In culture-based studies, three isolates were obtained. Two of these were related to Methanoculleus sp. IIE1 and one to Methanocalculus sp. 01F97C. These results clearly show that the Lonar Lake ecosystem harbors unexplored methanogens.


Similar content being viewed by others
References
Amann, RI, Ludwig, W, Schleifer, KI (1995) Phylogenetic identification and in situ detection of individual microbial cell without cultivation. Microbiol Rev 59: 143–155
American Public Health Association (APHA) (1992) In: Greenberg, A, Clesceri, L, Eaton, A (Eds.) Standard Methods for the Examination of Water and Wastewater, 18th edition, American Public Health Association, Washington
Boone, DR, Worakit, S, Mathrani, MI, Mah, RA (1986) Alkaliphilic methanogens from high-pH lake sediments. Syst Appl Microbiol 7: 230–234
DeLong, EF (1993) Archaea in coastal marine environments. Proc Natl Acad Sci USA 89: 5685–5689
Fredriksson, K, Dube, A, Milton, DJ, Balasundaram, MS (1976) Lonar Lake India: an impact crater in basalt. Science 180: 862–864
Grant, WD, Mwatha, WE, Jones, BE (1990) Alkaliphiles: ecology, diversity and application. FEMS Microbiol Rev 43: 260–296
Holdeman, LV, Moore, WEC (1972) Anaerobic Laboratory Manual. Virginia Polytechnic Institute and State University, Blacksburg, USA
Hungate, RE (1969) A roll tube method for cultivation of strict anaerobes. In: Norris, R, Ribbons, DW (Eds.), Methods in Microbiology, Vol, 3b. Academic Press Inc., New York, pp 117–132
Jones, BE, Grant, WD, Dockworth, AW, Owenson, GG (1998) Microbial diversity of soda lakes. Extremophiles 2: 191–200
Kadam, PC, Ranade, DR, Mandelco, L, Boone, DR (1994) Isolation and characterization of Methanolobus bombayensis sp. nov., a methylotrophic methanogen that requires high concentrations of divalent cations. Int J Syst Bacteriol 44: 603–607
Kevbrin, VV, Lysenko, AM, Zhilina, TN (1997) Physiology of Alkaliphilic methanogen Z-7936, a new strain of Methanosalsus zhilinaea isolated from Lake Magadi. Microbiology 66: 261–266
Lai, MC, Lin, CC, Yu, PH, Huang, YF, Chen, SC (2004) Methanocalculus chunghsingensis sp. nov., isolated from an estuary and a marine fishpond in Taiwan. Int J Syst Bacteriol 54: 183–189
Lai, M-C, Chen, S-C, Shu, C-M (2002) Methanocalculus taiwanensis sp. nov., isolated from an estuarine environment. Int J Syst Evol Microbiol 52: 1799–1806
Lane, DJ, Pace, B, Olsen, GJ, Stahl, DA, Sogin, ML, Pace, NR (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analysis. Proc Natl Acad Sci USA 82(20): 6955–6959
Litchfield, CD, Gillevet, PM (2002) Microbial diversity and complexity in hypersaline environments: a preliminary assessment. J Ind Microbiol Biotech 28: 48–55
Liu, Y, Boone, DR, Choy, C (1990) Methanohalophilus oregonense sp. nov., a methylotrophic methanogens from an alkaline, saline aquifer. Int J Syst Bacteriol 40: 111–116
Maidak, BL, Cole, JR, Parker, CT, Garrity, GM, Larsen, N, Li B, Lilburn, TG, MCCaughey, MJ, Olsen, GJ, Overbeek, R, Parmanik, S, Schmidt, TM, Tiedje, JM, Woese, CR (1999) A new version of the RDP (Ribosomal Database Project). Nucleic Acid Res 27: 171–173
Mathrani, IM, Boone, DR, Mah, RA, Fox, GE, Lau, PP (1988) Methanohalophilus zhilinae sp. nov. an alkaline, saline aquifer. Int J Syst Bacteriol 40: 111–116
Mikucki, JA, Liu, Y, Delwiche, M, Colwell, FS, Boone, DR (2003) Isolation of methanogens from deep marine sediments that contain methane hydrates, and description of Methanoculleus submarinus sp nov. Int J Syst Bacteriol 69: 3311–3316
Miller, TL, Wolin, MJ (1974) A serum bottle modification of Hungate technique for cultivation of strict anaerobes. Appl Microbiol 27: 985–987
Mori, K, Yamamoto, H, Kamagata, Y, Hatsu, M, Takamizawa, K (2000) Methanocalculus pumilus sp. nov., a heavy metal tolerant methanogen isolated from a waste-disposal site. Int J Syst Evol Microbiol 50: 1723–1729
Munson, MA, Needwell, DB, Embley, TM (1997) Phylogenetic diversity of Archaea in sediment samples from a coastal salt marsh. Appl Environ Microbiol 63: 4729–4733
Nandy, NC, Deo, VB (1961) Origin of the Lonar Lake and its Alkalinity. TISCO, pp 144–155
Posada, D, Crandall, KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14: 817–818
Ranade, DR, Gadre, RV (1988) Microbial Aspects of Anaerobic Digestion: Laboratory Manual. MACS Research Institute, Pune, India
Rees, HC, Grant, WD, Jones, BE, Heaphy, S (2003) Diversity of Kanyan Soda Lake alkaliphililes assessed by molecular methods. Extremophiles 8: 63–71
Ronquist, F, Huelsenbeck, JP (2003) MRBAYES 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574
Sambrook, J, Fritsch, EF, Maniatis, T (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor, NY
Sjoling, S, Cowan, DA (2003) High 16S rDNA bacterial diversity in glacial melt water lake sediment, Bratina Island, Antarctica. Exteremophiles 7: 275–282
Stackerbrandt, E, Goebel, B (1994) A place for DNA–DNA reassociation and 16S ribosomal-RNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol 44: 846–849
Thakker, CD, Ranade, DR (2002) Alkalophilic Methanosarcina isolated from Lonar Lake. Curr Sci 82: 455–458
Thompson, JD, Higgins, DJ, Gibson, TJ (1994) ClustalW: improving the sensitivity of multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acid Res 22: 4673–4680
Tonouchi, A (2004) Anaerobic 2-propanol degradation in anoxic paddy soil and the possible role of methanogens in its degradation. Curr Microbiol 49: 75–78
Williams, TG, Turpin, DH (1987) The role of external carbonic anhydrase in inorganic carbon acquisition by Chlamydomonas reinhardtii. Plant Physiol 83: 92–96
Wolin, EA, Wolin, MJ, Wolf, RS (1963) Formation of methane by bacteria extracts. J Biol Chem 238: 2882–2886
Wright, AG, Pimm, C (2003) Improved strategy for presumptive identification of methanogens using 16S riboprinting. J Microbiol Methods 55: 337–349
Yates, C, Gilling, MR, Davison, AD, Altavilla, N, Veal, DA (1997) PCR amplification of crude microbial DNA extracted from soil. Lett Appl Microbiol 25: 303–307
Zellner, G, Messner, P, Winter, J, Stackebrandt, E (1998) Methanoculleus palmolei sp. nov., an irregularly coccoid methanogen from an anaerobic digester treating wastewater of a palm oil plant in North-Sumatra, Indonesia. Int J Syst Bacteriol 48: 1111–1117
Zhilina, TN, Zavarzin, GA (1994) Alkaliphilic anaerobic consortium at pH 10. Curr Microbiol 29: 109–112
Zinder, SH, Salyers, AA (2001) Bergey’s manual of systematic bacteriology second edition vol 1: Microbial Ecology—new directions new importance, pp 101–110
Acknowledgment
The study was supported by a grant from Indian Space Research Organization (ISRO), Government of India as a part of the study of life in extreme environments. We also thank Mr. Dheeraj Dhotre (NCCS, Pune) for his help in phylogenetic analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Surakasi, V.P., Wani, A.A., Shouche, Y.S. et al. Phylogenetic Analysis of Methanogenic Enrichment Cultures Obtained from Lonar Lake in India: Isolation of Methanocalculus sp. and Methanoculleus sp.. Microb Ecol 54, 697–704 (2007). https://doi.org/10.1007/s00248-007-9228-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9228-z