Abstract
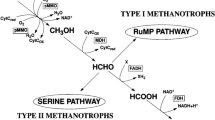
The enumeration of methanotrophic bacteria in the cover soil of an aged municipal landfill was carried out using (1) fluorescent in situ hybridization (FISH) with horseradish peroxidase-labeled oligonucleotide probes and tyramide signal amplification, also known as catalyzed reporter deposition-FISH (CARD-FISH), and (2) most probable number (MPN) method. The number of methanotrophs was determined in cover soil samples collected during April–November 2003 from a point with low CH4 emission. The number of types I and II methanotrophs obtained by CARD-FISH varied from 15 ± 2 to 56 ± 7 × 108 cells g−1 absolute dry mass (adm) of soil and methanotrophs of type I dominated over type II. The average number of methanotrophs throughout the cover soil profile was highest during May–September when the cover soil temperature was above 13°C. Methanotrophs accounted for about 50% of the total bacterial population in the deepest cover soil layer owing to higher availability of substrate (CH4). A lower number of methanotrophs (7 × 102 to 17 × 105 cells g−1 adm of soil) was determined by the MPN method compared to the CARD-FISH counts, thus confirming previous results that the MPN method is limited to the estimation of the culturable species that can be grown under the incubation conditions used. The number of culturable methanotrophs correlated with the methane-oxidizing activity measured in laboratory assays. In comparison to the incubation-based measurements, the number of methanotrophs determined by CARD-FISH better reflected the actual characteristics of the environment, such as release and uptake of CH4, temperature, and moisture, and availability of substrates.





Similar content being viewed by others
References
Adamsen, APS, King, GM (1993) Methane consumption in temperate and subarctic forest soils: rates, vertical zonation, and responses to water and nitrogen. Appl Environ Microbiol 59: 485–490
Alexander, M (1982) Most probable number method for microbial populations. In: Page, AL, Miller, RH, Keeney, DR (Eds.) Methods of soil analysis. Agronomy monograph No. 9, vol. 2, 2nd ed, American Society of Agronomy, Madison, Wisconsin, pp 815–820
Amann, RI, Binder, BJ, Olsen, RJ, Chisholm, SW, Devereux, R, Stahl, DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56: 1919–1925
Amann, RI, Zarda, B, Stahl, DA, Schleifer, KH (1992) Identification of individual prokaryotic cells by using enzyme-labeled, rRNA-targeted oligonucleotide probes. Appl Environ Microbiol 58: 3007–3011
Amaral, JA, Archambault, C, Richards, SR, Knowles, R (1995) Denitrification associated with groups I and II methanotrophs in a gradient enrichment system. FEMS Microbiol Ecol 18: 289–298
Boeckx, P, Van Cleemput, O (1996) Methane oxidation in neutral landfill cover soil: influence of moisture content, temperature, and nitrogen-turnover. J Environ Qual 25: 178–183
Bogner, JE, Spokas, KA, Burton, EA (1997) Kinetics of methane oxidation in a landfill cover soil: temporal variations, a whole-landfill oxidation experiment, and modeling of net CH4 emissions. Environ Sci Technol 31: 2504–2514
Bogner, JE, Spokas, KA, Burton, EA (1999) Temporal variations in greenhouse gas emissions at midlatitude landfill. J Environ Qual 28: 278–288
Börjesson, G, Sund, I, Svensson, B (2004) Microbial oxidation of CH4 at different temperatures in landfill cover soils. FEMS Microbiol Ecol 48: 305–312
Bourne, DG, Holmes, AJ, Iversen, N, Murrell, JC (2000) Fluorescent oligonucleotide rDNA probes for specific detection of methane oxidizing bacteria. FEMS Microbiol Ecol 31: 29–38
Bowman, J (2000) The methanotrophs—the families Methylococcaceae and Methilocystaceae. In: Dworkin, M et al (Ed.) The Prokaryotes: An evolving electronic resource for the microbiological community, 3rd edn, release 3.1
Chen, AC, Ueda, K, Sekiguchi, Y, Ohashi, A, Harada, H (2003) Molecular detection and direct enumeration of methanogenic Archaea and methanotrophic Bacteria in domestic solid waste landfill soils. Biotech Lett 25: 1563–1569
Christophersen, M, Linderød, L, Jensen, PE, Kjeldsen, P (2000) Methane oxidation at low temperatures in soil exposed to landfill gas. J Envirol Qual 29: 1989–1997
Czepiel, PM, Mosher, B, Crill, PM, Harriss, RC (1996) Quantifying the effect of oxidation on landfill methane emissions. J Geophys Res 101: 16721–16729
Dedysh, SN, Derakshani, M, Liesack, W (2001) Detection and enumeration of methanotrophs in acidic Sphagnum peat by 16S rRNA fluorescence in situ hybridization, including the use of newly developed oligonucleotide probes for Methylocella palustris. Appl Environ Microbiol 67: 4850–4857
Dedysh, SN, Smirnova, KV, Khmelenina, VN, Suzina, NE, Liesack, W, Trotsenko, YA (2005) Methylotrophic autotrophy in Beijerinckia mobilis. J Bacteriol 187: 3884–3888
Eller, G, Stubner, S, Frenzel, P (2001) Group-specific 16S rRNA targeted probes for the detection of type I and type II methanotrophs by fluorescent in situ hybridization. FEMS Microbiol Lett 198: 91–97
Escoffer, S, LeMer, J, Roger, PA (1997) Enumeration of methanotrophic bacteria in ricefield soils by plating and MPN techniques: a critical approach. Eur J Soil Biol 33: 41–51
Galchenko, VF, Shishkina, VN, Suzina, NE, Trotzenko, YA (1977) Isolation and properties of new strains of obligate methanotrophs. Microbiologiya 46: 890–897 (in Russian)
Galchenko, VF (2001) Methanotrophic bacteria. GEOS, Moscow, p 500 (in Russian)
Graham, DW, Chaudhary, JA, Hanson, RS, Arnold, RG (1993) Factors affecting competition between type I and type II methanotrophs in two-organism, continuous-flow reactors. Microb Ecol 25: 1–17
Gulledge, J, Ahmad, A, Steudler, PA, Pomerantz, WJ, Cavanaugh, CM (2001) Family- and genus-level 16S rRNA-targeted oligonucleotide probes for ecological studies of methanotrophic bacteria. Appl Environ Microbiol 67: 4726–4733
Hahn, D (2001) Manual for in situ analysis of microbial populations. In: Workshop on Sediments and Biofilms, 10–14 September 2001, EAWAG, Kastanienbaum, Switzerland
Hanson, RS, Hanson, TE (1996) Methanotrophic bacteria. Microbiol Rev 60: 439–471
IPCC (2001) Climate Change: The Scientific Bases. In: Houghton, JT, Ding, Y, Griggs, DJ, Noguer, M, Van der Linden, PJ, Day, X, Maskell, K, Johnson, CA (Eds.) Contribution of Working group I to the 3rd Assessment Report of the Intergovernmental Panel of Climate Change (2001), Cambridge University Press, Cambridge, p 881
Jones, HA, Nedwell, DB (1993) Methane emission and methane oxidation in landfill cover soil. FEMS Microbiol Ecol 102: 185–195
Kallistova, AYu, Kevbrina, MV, Nekrasova, VK, Glagolev, MV, Serebryanaya, MI, Nozhevnikova, AN (2005) Methane oxidation in landfill cover soil. Microbiology 74: 608–614
Kallistova, AYu, Glagolev, MV, Shnyrev, NA, Kevbrina, MV, Nekrasova, VK, Chistotin, MV, Phaustova, EV, Serebryanaya, MI, Nozhevnikova, AN (2006) Methane emission from the surface of the municipal solid waste landfill depending on landfill age and year season. J Ecol Chem 15: 13–21 (in Russian)
Kevbrina, MV, Okhapkina, AA, Akhlynin, DS, Kravchenko, IK, Nozhevnikova, AN, Galchenko, VF (2001) Growth of mesophilic methanotrophs at low temperatures. Microbiologiya 70: 444–451
Murrell, JC, McDonald, IR, Bourne, DG (1998) Molecular methods for the study of methanotroph ecology. FEMS Microbiol Ecol 27: 103–114
Nozhevnikova, AN, Lifshits, AB, Lebedev, VS, Zavarzin, GA (1993) Emission of methane into the atmosphere from landfills in the former USSR. Chemosphere 26: 401–417
Nozhevnikova, AN, Nekrasova, VK, Lebedev, VS, Lifshits, AB (1993) Microbiological processes in landfills. Water Sci Technol 27(2): 243–252
Nozhevnikova, A, Glagolev, M, Nekrasova, V, Einola, J, Sormunen, K, Rintala, J (2003) The analysis of methods for measurement of methane oxidation in landfills. Water Sci Technol 48(4): 45–52
Oda, Ya, Slagman, S-J, Meijer, WG, Forney, LJ, Gottschal, JC (2000) Influence of growth rate and starvation on fluorescent in situ hybridization of Rhodopseudomonas palustris. FEMS Microbiol Ecol 32: 205–213
Pernthaler A, Pernthaler J, Amann R (2002) Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl Environ Microbiol 68: 3094–3101
Schönhuber, W, Fuchs, B, Juretschko, S, Amann, RI (1997) Improved sensitivity of whole-cell hybridization by the combination of horseradish peroxidase- labeled oligonucleotides and tyramide signal amplification. Appl Environ Microbiol 63: 3268–3273
Stralis-Pavese, N, Sessitsch, A, Weilharter, A, Reichenauer, T, Riesing, J, Csontos, J, Murrell, JC, Bodrossy, L (2004) Optimization of diagnostic microarray for application in analyzing landfill methanotroph communities under different plant covers. Environ Microbiol 6: 347–363
Svenning, MM, Wartiainen, I, Hestnes, AG, Binnerup, SJ (2003) Isolation of methane oxidizing bacteria from soil by use of a soil substrate membrane system. FEMS Microbiol Ecol 44: 347–354
Tsien HC, Bratina BJ, Tsuji K, Hanson RS (1990) Use of oligodeoxynucleotide signature probes for identification of physiological groups of methylotrophic bacteria. Appl Environ Microbiol 56: 2858–2865
Tsubota, J, Eshinimaev, BT, Khmelenina, VN, Trotsenko, YA (2005) Methylothermus thermalis gen. nov., sp. nov., a novel moderately thermophilic obligate methanotroph from a hot spring in Japan. Int J Syst Evol Microbiol 55: 1877–1884
Wise, MG, McArthur, JV, Shimkets, LJ (1999) Methanotroph diversity in landfill soil: isolation of novel type I and type II methanotrophs whose presence was suggested by culture-independent 16S ribosomal DNA analysis. Appl Environ Microbiol 65: 4887–4897
Acknowledgments
We thank Dr. V. F. Galchenko for permission to use in this study the pure cultures of methanotrophic bacteria from his collection, S. A. Bykova for supplying these cultures, and Dr. I. K. Kravchenko for technical information. Special thanks to Dr. B. Wehrly for sending important reagents for the CARD-FISH and to F. Vazquez for methodological advice. We also thank M. V. Glagolev and M. V. Chistotin for help with field measurements and H. Wang for help with probe testing. This study was partially supported by INCO-COPERNICUS grant ICA2-CT-2001-10001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kallistova, A.Y., Kevbrina, M.V., Nekrasova, V.K. et al. Enumeration of Methanotrophic Bacteria in the Cover Soil of an Aged Municipal Landfill. Microb Ecol 54, 637–645 (2007). https://doi.org/10.1007/s00248-007-9219-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9219-0