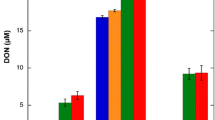
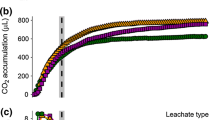
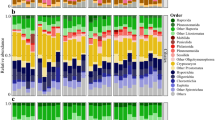
Abstract
Past studies have suggested that the concentration and quality of dissolved organic matter (DOM) may influence microbial community structure. In this study, we cross-inoculated the bacterial communities from two streams and a dystrophic lake that varied in DOM concentration and chemistry, to yield nine fully crossed treatments. We measured dissolved organic carbon (DOC) concentration and heterotrophic microbial community productivity throughout a 72-h incubation period, characterized DOM quality by molecular weight, and determined microbial community structure at the initial and final time points. Our results indicate that all bacterial inoculate sources had similar effects upon DOC concentration and DOM quality, regardless of the DOM source. These effects included an overall decrease in DOM M W and an initial period of DOC concentration variability between 0-24h. In contrast, microbial communities and their metabolic rates converged to profiles that reflected the DOM source upon which they were growing, regardless of the initial bacterial inoculation. The one exception was that the bacterial community from the low-concentration and low-molecular-weight DOM source exhibited a greater denaturing gradient gel electrophoresis (DGGE) band richness when grown in its own DOM source than when grown in the highest concentration and molecular weight DOM source. This treatment also exhibited a higher rate of productivity. In general, our data suggest that microbial communities are selected by the DOM sources to which they are exposed. A microbial community will utilize the low-molecular-weight (or labile) DOM sources as well as parts of the high-molecular-weight (refractory) DOM, until a community develops that can efficiently metabolize the more abundant high-molecular-weight source. This experiment examines some of the complex interactions between microbial community selection and the combined factors of DOM quality and concentration. Our data suggest that the roles of aerobic aquatic heterotrophic bacteria in carbon cycling, as well as the importance of high-molecular-weight DOM as a carbon source, may be more complex than is conventionally recognized.



Similar content being viewed by others
References
Allard, B, Borén, H, Pettersson, C, Zhang, G (1994) Degradation of humic substances by UV irradiation. Environ Int 20: 97–101
Anesio, AM, Hollas, C, Granéli, W, Laybourn-Parry, J (2004) Influence of humic substances on bacterial and viral dynamics in freshwaters. Appl Environ Microbiol 70(8): 4848–4854
APHA (1992) Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington, DC
Burkert, U, Warnecke, F, Babenzien, D, Zwirnmann, E, Pernthaler, J (2003) Members of a readily enriched β-Proteobacterial clade are common in surface waters of a humic lake. Appl Environ Microbiol 69(11): 6550–6559
Chin, Y-P, Aiken, G, O'Loughlin, E (1994) Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ Sci Technol 28: 1853–1858
Cottrell, MT, Kirchman, DL (2000) Natural assemblages of marine Proteobacteria and members of the Cytophaga–flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl Environ Microbiol 66(4): 1692–1697
Eiler, A, Langenheder, S, Bertilsson, S, Tranvik, LJ (2003) Heterotrophic bacterial growth efficiency and community structure at different natural organic carbon concentrations. Appl Environ Microbiol 69(7): 3701–3709
Ellis, BD, Butterfield, P, Jones, WL, McFeters, GA, Camper, AK (2000) Effects of carbon source, carbon concentration, and chlorination on growth related parameters of heterotrophic biofilm bacteria. Microb Ecol 38: 330–347
Findlay, SEG, Sinsabaugh, RL, Sobczak, WV, Hoostal, M (2003) Metabolic and structural response of hyporheic microbial communities to variations in supply of dissolved organic matter. Limnol Oceanogr 48(4): 1608–1617
Fischer, H, Sachse, A, Steinberg, CEW, Pusch, M (2002) Differential retention and utilization of dissolved organic carbon by bacteria in river sediments. Limnol Oceanogr 47(6): 1702–1711
Fisher, MM, Klug, JL, Lauster, G, Newton, M, Triplett, EW (2000) Effects of resources and trophic interactions on freshwater bacterioplankton diversity. Microb Ecol 40: 125–138
Frost, PC, Maurice, PA, Fein, JB (2003) The effect of cadmium on fulvic acid adsorption to Bacillus subtilis. Chem Geol 200: 217–224
Gayte, X, Fontvieille, D, Wilkinson, KJ (1999) Bacterial stimulation in mixed cultures of bacteria and organic carbon from river and lake waters. Microb Ecol 38: 285–295
Hobbie, JE, Daley, J, Jasper, S (1977) Use of Nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33: 1225–1228
Kirchman, DL (1993) Leucine incorporation as a measure of biomass production. In: Kemp, PF, Sherr, BF, Sherr, EB, Cole, JJ (Eds.) Handbook of Methods in Aquatic Microbial Ecology. Lewis Publishers, Boca Raton, fl, pp 509–512
Kisand, V, Wikner, J (2003) Combining culture-dependent and -independent methodologies for estimation of richness of estuarine bacterioplankton consuming riverine dissolved organic matter. Appl Environ Microbiol 69(6): 3607–3616
Kopáček, J, Hejzlar, J, Kaňa, J, Porcal, P, Klementová, S (2003) Photochemical, chemical, and biological transformations of dissolved organic carbon and its effect on alkalinity production in acidified lakes. Limnol Oceanogr 48(1): 106–117
Kritzberg, ES, Cole, JJ, Pace, ML, Granéli, W, Bade, DL (2004) Autochthonous versus allochthonous carbon sources of bacteria: results from whole-lake 13C addition experiments. Limnol Oceanogr 49(2): 588–596
Mann, CJ, Wetzel, RG (1995) Dissolved organic carbon and its utilization in a riverine wetland ecosystem. Biogeochemistry 31: 99–120
Maurice, PA, Manecki, M, Fein, JB, Schaefer, J (2004) Fractionation of an aquatic fulvic acid upon adsorption to the bacterium, Bacillus subtilis. Geomicrobiol J 21: 1–10
Meyer, JL, Edwards, RT, Risley, R (1987) Bacterial growth on dissolved organic carbon from a blackwater river. Microb Ecol 13: 13–29
Moran, MA, Hodson, RE (1990) Bacterial production on humic and nonhumic components of dissolved organic carbon. Limnol Oceanogr 35(8): 1744–1756
Muyzer, G, de Waal, EC, Uitterlinden, AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59: 695–700
Myers, RM, Fischer, SG, Lerman, LS, Maniatis, T (1985) Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res 13: 3131–3145
Obernosterer, I, Benner, R (2004) Competition between biological and photochemical processes in the mineralization of dissolved organic carbon. Limnol Oceanogr 49(1): 117–124
Ogawa, H, Amagai, Y, Koike, I, Kaiser, K, Benner, R (2001) Production of refractory dissolved organic matter by bacteria. Science 292: 917–920
Olsen, LM, Reinertsen, H, Vadstein, O (2002) Can phosphorus limitation inhibit dissolved organic carbon consumption in aquatic microbial food webs? A study of three food web structures in mesocosms. Microb Ecol 43: 353–366
Smith, EM, Prairie, YT (2004) Bacterial metabolism and growth efficiency in lakes: the importance of phosphorus availability. Limnol Oceanogr 49(1): 137–147
Strauss, EA, Lamberti, GA (2002) Effect of dissolved organic carbon quality on microbial decomposition and nitrification rates in stream sediments. Freshw Biol 47: 65–74
Throbäck, IN, Enwall, K, Jarvis, A, Hallin, S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol 49: 401–417
Traina, SJ, Novak, J, Smeck, NE (1990) An ultraviolet absorbance method of estimating the percent aromatic carbon content of humic acids. J Environ Qual 19: 151–153
Wehr, JD, Petersen, J, Findlay, S (1999) Influence of three contrasting detrital carbon sources on planktonic bacterial metabolism in a mesotrophic lake. Microb Ecol 37: 23–35
Wiegner, TN, Seitzinger, SP (2004) Season bioavailability of dissolved organic carbon and nitrogen from pristine and polluted freshwater wetlands. Limnol Oceanogr 49(5): 1703–1712
Yokokawa, T, Nagata, T, Cottrell, MT, Kirchman, DL (2004) Growth rate of the major phylogenetic bacterial groups in the Delaware estuary. Limnol Oceanogr 49(5): 1620–1629
Young, KC, Docherty, KM, Maurice, PA, Bridgham, SD (2005) Dissolved organic matter biodegradation in two streams and a dystrophic pond: influence of source and native microbial population. Hydrobiologia 539: 1–11
Young, KC, Maurice, PA, Docherty, KM, Bridgham, SD (2004) Bacterial degradation of dissolved organic matter from two northern Michigan streams. Geomicrobiol J 21: 521–528
Ziegler, SE, Fogel, ML (2003) Seasonal and diel relationships between the isotopic compositions of dissolved and particulate organic matter in freshwater ecosystems. Biogeochemistry 64: 25–52
Zou, L, Wang, X, Callahan, J, Culp, RA, Chen, RF, Altabet, MA, Sun, M (2004) Bacterial roles in the formation of high-molecular-weight dissolved organic matter in estuarine and coastal waters: evidence from lipids and the compound-specific isotopic ratios. Limnol Oceanogr 49(1): 297–302
Acknowledgment
We would like to thank the National Science Foundation Graduate Research Fellowship Program, the Hydrologic Sciences Division of the National Science Foundation, and the Environmental Protection Agency Science and Achieve Results (STAR) program. Although the research described in this article has been funded in part by the United States Environmental Protection Agency, it has not been subjected to the Agency's required peer and policy review and, therefore, does not necessarily reflect the views of the Agency and no official endorsement should be inferred. We would like to thank the University of Notre Dame's Environmental Research Center and Center for Environmental Science and Technology, Dr. Charles F. Kulpa Jr., Dennis Birdsell, and James Larson for logistical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Docherty, K.M., Young, K.C., Maurice, P.A. et al. Dissolved Organic Matter Concentration and Quality Influences upon Structure and Function of Freshwater Microbial Communities. Microb Ecol 52, 378–388 (2006). https://doi.org/10.1007/s00248-006-9089-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-006-9089-x