Abstract
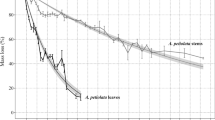
Decomposition of culms (sheaths and stems) of the emergent macrophyte Phragmites australis (common reed) was followed for 16 months in the litter layer of a brackish tidal marsh along the river Scheldt (the Netherlands). Stems and leaf sheaths were separately analyzed for mass loss, litter-associated fungal biomass (ergosterol), nutrient (N and P), and cell wall polymer concentrations (cellulose and lignin). The role of fungal biomass in litter nutrient dynamics was evaluated by estimating nutrient incorporation within the living fungal mass. After 1 year of standing stem decay, substantial fungal colonization was found. This corresponded to an overall fungal biomass of 49 ± 8.7 mg g−1 dry mass. A vertical pattern of fungal colonization on stems in the canopy is suggested. The litter bag experiment showed that mass loss of stems was negligible during the first 6 months, whereas leaf sheaths lost almost 50% of their initial mass during that time. Exponential breakdown rates were −0.0039 ± 0.0004 and −0.0026 ± 0.0003 day−1 for leaf sheaths and stems, respectively (excluding the initial lag period). In contrast to the stem tissue—which had no fungal colonization—leaf sheaths were heavily colonized by fungi (93 ± 10 mg fungal biomass g−1 dry mass) prior to placement in the litter layer. Once being on the sediment surface, 30% of leaf sheath's associated fungal biomass was lost, but ergosterol concentrations recovered the following months. In the stems, fungal biomass increased steadily after an initial lag period to reach a maximal biomass of about 120 mg fungal biomass g−1 dry mass for both plant parts at the end of the experiment. Fungal colonizers are considered to contain an important fraction of nutrients within the decaying plant matter. Fungal N incorporation was estimated to be 64 ± 13 and 102 ± 15% of total available N pool during decomposition for leaf sheaths and stems, respectively. Fungal P incorporation was estimated to be 37 ± 9 and 52 ± 15% of total available P during decomposition for leaf sheaths and stems, respectively. Furthermore, within the stem tissue, fungi are suggested to be active immobilizers of nutrients from the external environment because fungi were often estimated to contain more than 100% of the original nutrient stock.







Similar content being viewed by others
References
Apinis, AE, Chesters, CGC, Taligoola, HK (1972) Colonisation of Phragmites communis leaves by fungi. Nova Hedwig 23: 113–124
Apinis, AE, Chesters, CGC, Taligoola, HK (1975) Microfungi colonizing nodes and internodes of aerial standing culms of Phragmites communis Trin. Nova Hedwig 26: 495–507
Beever, RE, Burns, DJW (1980) Phosphorus uptake, storage and utilization by fungi. Adv Bot Res 8: 127–219
Bergbauer, M, Newell, SY (1992) Contribution to lignocellulose degradation and DOC formation from a salt marsh macrophyte by the ascomycete Phaeosphaeria spartinicola. FEMS Microb Ecol 86: 341–348
Bergbauer, M, Moran, MA, Hodson, RE (1992) Decomposition of lignocellulose from a freshwater macrophyte by aero-aquatic fungi. Microb Ecol 23: 159–167
Boulton, AJ, Boon, PI (1991) A review of methodology used to measure leaf litter decomposition in lotic environments: time to turn over an old leaf? Aust J Mar Freshwater Res 42: 1–43
Dowding, P (1981) Nutrient uptake and allocation during substrate exploitation by fungi. In: Wicklow, DT, Caroll, GC (Eds.), The Fungal Community: Its Organization and Role in the Ecosystem. Marcel Dekker. New York, pp 621–635
Du Laing, G, Van Ryckegem, G, Tack, FMG, Verloo, MG (2005) Metal accumulation in intertidal litter through decomposing leaf blades, sheaths and stems of Phragmites australis. Chemosphere (in press). DOI 10.1016/j.chemosphere.2005.10.034
Findlay, S, Howe, K, Austin, HK (1990) Comparison of detritus dynamics in two tidal freshwater wetlands. Ecology 71: 288–295
Findlay, SEG, Dye, S, Kuehn, KA (2002) Microbial growth and nitrogen retention in litter of Phragmites australis compared to Typha angustifolia. Wetlands 22: 616–625
Gessner, MO (2000) Breakdown and nutrient dynamics of submerged Phragmites shoots in the littoral zone of a temperate hardwater lake. Aquat Bot 66: 9–20
Gessner, MO (2001) Mass loss, fungal colonisation and nutrient dynamics of Phragmites australis leaves during senescence and early decay. Aquat Bot 69: 325–339
Gessner, MO, Chauvet, E (1994) Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75: 1807–1817
Gessner, MO, Schmitt, AL (1996) Use of solid-phase extraction to determine ergosterol concentrations in plant tissue colonized by fungi. Appl Environ Microbiol 62: 415–419
Gessner, MO, Van Ryckegem, G (2003) Water fungi as decomposers in freshwater ecosystems. In: Bitton, G (Ed.) Encyclopedia of Environmental Microbiology. Wiley, New York. Online edition: DOI 10.1002/0471263397.env314
Gessner, MO, Schieferstein, B, Müller, U, Barkmann, S, Lenfers, UA (1996) A partial budget of primary organic carbon flows in the littoral zone of a hardwater lake. Aquat Bot 55: 93–105
Gessner, MO, Suberkropp, K, Chauvet, E (1997) Decomposition of plant litter by fungi in marine and freshwater ecosystems. In: Wicklow, DC, Söderström, B (Eds.) The Mycota IV, Environmental and Microbial Relationships. Springer. Berlin, pp 303–322
Granéli, W (1990) Standing crop and mineral content of reed, Phragmites australis (Cav.) Trin. ex Steud., in Sweden—management of reed stands to maximize harvestable biomass. Folia Geobot Phytotaxon 25: 291–302
Grattan II, RM, Suberkropp, K (2001) Effects of nutrient enrichment on yellow poplar leaf decomposition and fungal activity in streams. J North Am Benthol Soc 20: 33–43
Haslam, SM (1972) Biological flora of the British Isles no. 128, Phragmites communis Trin. J Ecol 60: 585–610
Hatfield, RD, Jung, H-JG, Ralph, J, Buxton, DR, Weimer, PJ (1994) A comparison of the insoluble residues produced by the Klason lignin and acid detergent lignin procedures. J Sci Food Agric 65: 51–58
Hietz, P (1992) Decomposition and nutrient dynamics of reed (Phragmites australis (Cav.) Trin. ex Steud.) litter in Lake Neusiedl, Austria. Aquat Bot 43: 211–230
Jones, HL, Worrall, JJ (1995) Fungal biomass in decayed wood. Mycologia 87: 459–466
Jung, H-JG, Varel, VH, Weimer, PJ, Ralph, J (1999) Accuracy of Klason lignin and acid detergent lignin methods as assessed by bomb calorimetry. J Agric Food Chem 47: 2005–2008
Kirk, TK, Obst, JR (1988) Lignin determination. Methods Enzymol 161: 87–101
Komínková, D, Kuehn, KA, Büsing, N, Steiner, D, Gessner, MO (2000) Microbial biomass, growth, and respiration associated with submerged litter of Phragmites australis decomposing in a littoral reed stand of a large lake. Aquat Microb Ecol 22: 271–282
Kuehn, KA, Gessner, MO, Wetzel, RG, Suberkropp, K (1999) Decomposition and CO2 evolution from standing litter of the emergent macrophyte Erianthus giganteus. Microb Ecol 38: 50–57
Kuehn, KA, Lemke, MJ, Suberkropp, K, Wetzel, RG (2000) Microbial biomass and production associated with decaying leaf litter of the emergent macrophyte Juncus effesus. Limnol Oceanogr 45: 862–870
Kuehn, KA, Steiner, D, Gessner, MO (2004) Diel mineralization patterns of standing–dead plant litter: implications for CO2 flux from wetlands. Ecology 85: 2504–2518
Meganck, B (1998) The morphology of Phragmites australis (Cav.) Steud. in relation to hydrodynamics, management and salinity in the Scheldt estuary. Unpublished master's thesis (in Dutch), Ghent University, Ghent, Belgium
Mille-Lindblom, C, von Wachenfeldt, E, Tranvik, LJ (2004) Ergosterol as a measure of living fungal biomass: persistence in environmental samples after fungal death. J Microbiol Methods 59: 253–262
Mitsch, WJ, Gosselink, JG (2000) Wetlands, 3rd edn. Van Nostrand Reinhold. New York
Newell, SY (1992) Estimating fungal biomass and productivity in decomposing litter. In: Caroll, GC, Wicklow, DT (Eds.) The Fungal Community: Its Organisation and Role in the Ecosystem, 2nd. Marcel Dekker. New York, pp 521–561
Newell, SY (1996) Established and potential impacts of eukaryotic mycelial decomposers in marine/terrestrial ecotones. J Exp Mar Biol Ecol 200: 187–206
Newell, SY (2001) Spore-expulsion rates and extents of blade occupation by Ascomycetes of the smooth-cordgrass standing-decay system. Bot Mar 44: 277–285
Newell, SY, Arsuffi, TL, Palm, LA (1996) Misting and nitrogen fertilization of shoots of a saltmarsh grass: effects upon fungal decay of leaf blades. Oecologia 108: 495–502
Newell, SY, Fallon, RD, Miller, JD (1989) Decomposition and microbial dynamics for standing, naturally positioned leaves of the salt-marsh grass Spartina alterniflora. Mar Biol 101: 471–481
Newell, SY, Moran, MA, Wicks, R, Hodson, RE (1995) Procutivities of microbial decomposers during early stages of decomposition of leaves of a freshwater sedge. Freshwater Biol 34: 135–148
Newell, SY, Wasowski, J (1995) Sexual productivity and spring intramarsh distribution of a key saltmarsh microbial sec. producer. Estuaries 18: 241–249
Palm, CA, Rowland, AP (1997) A minimum dataset for characterization of plant quality for decomposition. In: Cadisch, G, Giller, KE (Eds.) Driven by Nature: Plant Litter Quality and Decomposition. CAB International. Oton, pp 379–392
Pieczyńska, E (1972) Ecology of the eulittoral zone of lakes. Ekol Pol 20: 637–732
Polunin, NVC (1982) Processes contributing to the decay of reed (Phragmites australis) litter in freshwater. Arch Hydrobiol 94: 182–209
Polunin, NVC (1984) The decomposition of emergent macrophytes in fresh water. Adv Ecol Res 14: 115–166
Rodewald, L, Rudescu, L (1974) Das Schilfrohr. Binnengewässer 27: 1–302
Soetaert, K, Hoffmann, M, Meire, P, Starink, M, van Oevelen, D, Van Regenmortel, S, Cox, T (2004) Modeling growth and carbon allocation in two reed beds (Phragmites australis) in the Scheldt estuary. Aquat Bot 79: 211–234
Suberkropp, K (1995) The influence of nutrients on fungal growth, productivity, and sporulation during leaf breakdown in streams. Can J Bot Suppl 1: 1361–1369
Suberkropp, K, Chauvet, E (1995) Regulation of leaf breakdown by fungi in streams: Influences of water chemistry. Ecology 76: 1433–1445
Tanaka, Y (1991) Microbial decomposition of reed (Phragmites communis) leaves in a saline lake. Hydrobiologia 220: 119–129
Tanaka, Y (1993) Aerobic cellulolytic bacterial-flora associated with decomposing Phragmites leaf-litter in a seawater lake. Hydrobiologia 263: 145–154
Van Damme, S, Meire, P, Maeckelberghe, H, Verdievel, M, Bourgoing, L, Taverniers, E, Ysebaert, T, Wattel, G (1995) De waterkwaliteit van de Zeeschelde: evolutie in de voorbije dertig jaar. Water 85: 244–256
Van Damme, S, Ysebaert, T, Meire, P, Van den Bergh, E (1999) Habitatstructuren, waterkwaliteit en leefgemeenschappen in het Schelde-estuarium. Rapport IN 99/24. Instituut voor Natuurbehoud, Brussels
Van Ryckegem, G (2005) Fungi on common reed (Phragmites australis): fungal diversity, community structure and decomposition processes. Ph.D. thesis. Ghent University, Belgium, pp 424
Van Ryckegem, G, Gessner, MO, Verbeken, A (2006) Fungi on leaf blades of Phragmites australis in a brackish tidal marsh: Diversity, succession, and leaf decomposition. Microb Ecol
Van Ryckegem, G, Verbeken, A (2005) Fungal diversity and community structure on Phragmites australis (Poaceae) along a salinity gradient in the Scheldt estuary (Belgium). Nova Hedwig 80: 173–197
Van Ryckegem, G, Verbeken, A (2005) Fungal ecology and succession on Phragmites australis in a brackish tidal marsh. I. Leaf sheaths. Fungal Div 19: 157–187
Van Ryckegem, G, Verbeken, A (2005) Fungal ecology and succession on Phragmites australis in a brackish tidal marsh. II. Stems. Fungal Div 20: 209–233
Van Soest, PJ, Robertson, JB (1980) Systems of analysis for evaluation fibrous feeds. In: Pigden, WJ, Balch, CC, Graham, M (Eds.) Standardization of Analytical Methodology for Feeds. International Development Center. Ottawa, pp 49–60
Van Soest, PJ, Wine, RH (1967) Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J Assoc Off Anal Chem 50: 50–55
Webster, J (1956) Succession of fungi on decaying cocksfoot culms. I. J Ecol 44: 517–544
Webster, JR, Benfield, EF (1986) Vascular plant breakdown in freshwater ecosystems. Annu Rev Ecol Syst 17: 567–594
Acknowledgments
We thank R. Heynderickx and A. Van Heuverswijn for assistance with making litter bags and Dirk Van Gansbeke (Ghent University, Laboratory of Marine Biology) for nitrogen and phosphorus analyses. Prof. Dr. Mark Stevens (Ghent University, Laboratory of Wood Technology) kindly offered facilities to perform acid detergent fiber analysis. Bernhard De Meyer is warmly acknowledged for practical assistance during lignin determination. Thanks to Steven Newell for commenting on a previous version of this manuscript. This research was funded by the Institute for the Promotion of Innovation by Science and Technology in Flanders, Belgium, through a grant to G.V.R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Ryckegem, G., Van Driessche, G., Van Beeumen, J.J. et al. The Estimated Impact of Fungi on Nutrient Dynamics During Decomposition of Phragmites australis Leaf Sheaths and Stems. Microb Ecol 52, 564–574 (2006). https://doi.org/10.1007/s00248-006-9003-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-006-9003-6