Abstract
Background
Neonatal hypoxic-ischemic encephalopathy (HIE) is one of the common causes of neurological injury in full-term neonates following perinatal asphyxia. The conventional magnetic resonance technique has low sensitivity in detecting variations in cerebral blood flow in patients with HIE.
Objective
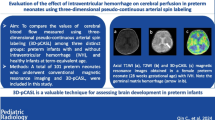
This article evaluates the clinical diagnostic value of three-dimensional pseudo-continuous arterial spin labelling (3-D pcASL) perfusion magnetic resonance imaging (MRI) for early prediction of neurobehavioral outcomes in full-term neonates with HIE.
Materials and methods
All neonates diagnosed with HIE underwent MRI (conventional and 3-D pcASL perfusion MRI). Cerebral blood flow values were measured in the basal ganglia (caudate nuclei, lenticular nuclei), thalami and white matter regions (frontal lobes, corona radiata). After 1-month follow-up, the Neonatal Behavioral Neurological Assessment scores were used to divide patients into favourable outcome group versus adverse outcome group.
Results
Twenty-three patients were enrolled in this study. There were no statistical differences between the symmetrical cerebral blood flow values of bilateral basal ganglia, thalami and white matter regions. However, the cerebral blood flow values of grey matter nuclei were higher than the white matter regions. The average value of cerebral blood flow in the basal ganglia and thalami in the adverse outcome group was 37.28±6.42 ml/100 g/min, which is greater than the favourable outcome group (22.55 ± 3.21 ml/100 g/min) (P<0.01). The area under the curve (AUC) of 3-D pcASL perfusion MRI was 0.992 with a cutoff value of 28.75 ml/100 g/min, with a Youden’s index of 0.9231. The sensitivity and specificity were 92.3% and 100%, respectively.
Conclusion
The 3-D pcASL demonstrated higher perfusion alteration in the basal ganglia and thalami of neonatal HIE with adverse outcomes. The 3-D pcASL perfusion MRI has the potential to predict neurobehavioral outcomes of neonates with HIE.





Similar content being viewed by others
References
(1996) Use and abuse of the Apgar score. Committee on Fetus and Newborn, American Academy of Pediatrics, and Committee on Obstetric Practice, American College of Obstetricians and Gynecologists. Pediatrics 98:141–142
Imai K, de Vries LS, Alderliesten T et al (2018) MRI changes in the thalamus and basal ganglia of full-term neonates with perinatal asphyxia. Neonatology 114:253–260
O'Sullivan MP, Looney AM, Moloney GM et al (2019) Validation of altered umbilical cord blood microRNA expression in neonatal hypoxic-ischemic encephalopathy. JAMA Neurol 76:333–341
Volpe JJ (2012) Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann Neurol 72:156–166
Wong AM-C, Yeh C-H, Liu H-L et al (2017) Arterial spin-labeling perfusion imaging of children with subdural hemorrhage: perfusion abnormalities in abusive head trauma. J Neuroradiol 44:281–287
Lee YK, Penn A, Patel M et al (2017) Hypothermia-treated neonates with hypoxic-ischemic encephalopathy: optimal timing of quantitative ADC measurement to predict disease severity. Neuroradiol J 30:28–35
Seo Y, Kim G-T, Choi JW (2017) Early detection of neonatal hypoxic-ischemic white matter injury: an MR diffusion tensor imaging study. Neuroreport 28:845–855
Guo L, Wang D, Bo G et al (2016) Early identification of hypoxic-ischemic encephalopathy by combination of magnetic resonance (MR) imaging and proton MR spectroscopy. Exp Ther Med 12:2835–2842
Finder M, Boylan GB, Twomey D et al (2020) Two-year neurodevelopmental outcomes after mild hypoxic ischemic encephalopathy in the era of therapeutic hypothermia. JAMA Pediatr 174:48–55
Massaro AN, Bouyssi-Kobar M, Chang T et al (2013) Brain perfusion in encephalopathic newborns after therapeutic hypothermia. AJNR AmJ Neuroradiol 34:1649–1655
Varela M, Petersen ET, Golay X, Hajnal JV (2015) Cerebral blood flow measurements in infants using look-locker arterial spin labeling. J Magn Reson Imaging 41:1591–1600
De Vis JB, Petersen ET, Alderliesten T et al (2014) Non-invasive MRI measurements of venous oxygenation, oxygen extraction fraction and oxygen consumption in neonates. Neuroimage 95:185–192
Wintermark P, Hansen A, Gregas MC et al (2011) Brain perfusion in asphyxiated newborns treated with therapeutic hypothermia. AJNR Am J Neuroradiol 32:2023–2029
Tang S, Liu X, He L et al (2019) Application of a 3D pseudocontinuous arterial spin-labeled perfusion MRI scan combined with a postlabeling delay value in the diagnosis of neonatal hypoxic-ischemic encephalopathy. PLoS One 14:e0219284
Wang J-N, Li J, Liu H-J et al (2019) Application value of three-dimensional arterial spin labeling perfusion imaging in investigating cerebral blood flow dynamics in normal full-term neonates. BMC Pediatr 19:495
De Vis JB, Hendrikse J, Petersen ET et al (2015) Arterial spin-labelling perfusion MRI and outcome in neonates with hypoxic-ischemic encephalopathy. Eur Radiol 25:113–121
Pienaar R, Paldino MJ, Madan N et al (2012) A quantitative method for correlating observations of decreased apparent diffusion coefficient with elevated cerebral blood perfusion in newborns presenting cerebral ischemic insults. Neuroimage 63:1510–1518
Zakariaee SS, Oghabian MA, Firouznia K et al (2018) Assessment of the agreement between cerebral hemodynamic indices quantified using dynamic susceptibility contrast and dynamic contrast-enhanced perfusion magnetic resonance imagings. J Clin Imaging Sci 8:2
Wong SM, Jansen JFA, Zhang CE et al (2019) Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology 92:e1669–e1677
Alsop DC, Detre JA, Golay X et al (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116
Hu HH, Rusin JA, Peng R et al (2019) Multi-phase 3D arterial spin labeling brain MRI in assessing cerebral blood perfusion and arterial transit times in children at 3T. Clin Imaging 53:210–220
Hu HH, Li Z, Pokorney AL et al (2017) Assessment of cerebral blood perfusion reserve with acetazolamide using 3D spiral ASL MRI: preliminary experience in pediatric patients. Magn Reson Imaging 35:132–140
Shang S, Wu J, Zhang H et al (2021) Motor asymmetry related cerebral perfusion patterns in Parkinson's disease: an arterial spin labeling study. Human Brain Mapp 42:298–309
Cowan F, Rutherford M, Groenendaal F et al (2003) Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 361:736–742
Group of Neonatology, Chinese Pediatric Society, Chinese Medical Association (2005) Diagnostic criteria for neonatal hypoxic-ischemic encephalopathy. Zhonghua Er Ke Za Zhi 43:584
Bao XL, Yu RJ, Li ZS, Zhang BL (1991) Twenty-item behavioral neurological assessment for normal newborns in 12 cities of China. Chin Med J 104:742–746
Bao XL, Yu RJ, Li ZS (1993) 20-item neonatal behavioral neurological assessment used in predicting prognosis of asphyxiated newborn. Chin Med J 106:211–215
Alderliesten T, de Vries LS, Benders MJNL et al (2011) MR imaging and outcome of term neonates with perinatal asphyxia: value of diffusion-weighted MR imaging and 1H MR spectroscopy. Radiology 261:235–242
Vermeulen RJ, van Schie PEM, Hendrikx L, Barkhof F (2008) Diffusion-weighted and conventional MR imaging in neonatal hypoxic ischemia: two-year follow-up study. Radiology 249:631–639
Geva S, Jentschke S, Argyropoulos GPD et al (2020) Volume reduction of caudate nucleus is associated with movement coordination deficits in patients with hippocampal atrophy due to perinatal hypoxia-ischaemia. Neuroimage Clin 28:102429
Boichot C, Walker PM, Durand C et al (2006) Term neonate prognoses after perinatal asphyxia: contributions of MR imaging, MR spectroscopy, relaxation times, and apparent diffusion coefficients. Radiology 239:839–848
Thayyil S, Chandrasekaran M, Taylor A et al (2010) Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 125:e382–e395
L'Abee C, de Vries LS, van der Grond J, Groenendaal F (2005) Early diffusion-weighted MRI and 1H-magnetic resonance spectroscopy in asphyxiated full-term neonates. Biol Neonate 88:306–312
Wang H, Li S, Chen X et al (2020) Cerebral blood flow alterations in high myopia: an arterial spin labeling study. Neural Plast 2020:6090262
Munsch F, Taso M, Zhao L et al (2020) Rotated spiral RARE for high spatial and temporal resolution volumetric arterial spin labeling acquisition. Neuroimage 223:117371
Varela M, Hajnal JV, Petersen ET et al (2011) A method for rapid in vivo measurement of blood T1. NMR Biomed 24:80–88
Parkes LM, Rashid W, Chard DT, Tofts PS (2004) Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 51:736–743
Wassink G, Gunn ER, Drury PP et al (2014) The mechanisms and treatment of asphyxial encephalopathy. Front Neurosci 8:40
Bennet L, Roelfsema V, Pathipati P et al (2006) Relationship between evolving epileptiform activity and delayed loss of mitochondrial activity after asphyxia measured by near-infrared spectroscopy in preterm fetal sheep. J Physiol 572:141–154
Kleuskens DG, Goncalves Costa F, Annink KV et al (2021) Pathophysiology of cerebral hyperperfusion in term neonates with hypoxic-ischemic encephalopathy: a systematic review for future research. Front Pediatr 9:631258
Bennet L, Tan S, Van den Heuij L et al (2012) Cell therapy for neonatal hypoxia-ischemia and cerebral palsy. Ann Neurol 71:589–600
de Vries LS, Groenendaal F (2010) Patterns of neonatal hypoxic-ischaemic brain injury. Neuroradiol 52:555–566
Barkovich AJ, Miller SP, Bartha A et al (2006) MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol 27:533–547
De Vis JB, Hendrikse J, Groenendaal F et al (2014) Impact of neonate haematocrit variability on the longitudinal relaxation time of blood: implications for arterial spin labelling MRI. Neuroimage Clin 4:517–525
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, J., Mu, Y., Xu, X. et al. Cerebral perfusion changes of the basal ganglia and thalami in full-term neonates with hypoxic-ischaemic encephalopathy: a three-dimensional pseudo continuous arterial spin labelling perfusion magnetic resonance imaging study. Pediatr Radiol 52, 1559–1567 (2022). https://doi.org/10.1007/s00247-022-05344-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-022-05344-4