Abstract
Background
Germinal matrix hemorrhage–intraventricular hemorrhage is among the most common intracranial complications in premature infants. Early detection is important to guide clinical management for improved patient prognosis.
Objective
The purpose of this study was to assess whether a convolutional neural network (CNN) can be trained via transfer learning to accurately diagnose germinal matrix hemorrhage on head ultrasound.
Materials and methods
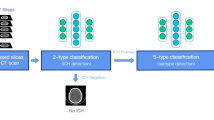
Over a 10-year period, 400 head ultrasounds performed in patients ages 6 months or younger were reviewed. Key sagittal images at the level of the caudothalamic groove were obtained from 200 patients with germinal matrix hemorrhage and 200 patients without hemorrhage; all images were reviewed by a board-certified pediatric radiologist. One hundred cases were randomly allocated from the total for validation and an additional 100 for testing of a CNN binary classifier. Transfer learning and data augmentation were used to train the model.
Results
The median age of patients was 0 weeks old with a median gestational age of 30 weeks. The final trained CNN model had a receiver operating characteristic area under the curve of 0.92 on the validation set and accuracy of 0.875 on the test set, with 95% confidence intervals of [0.86, 0.98] and [0.81, 0.94], respectively.
Conclusion
A CNN trained on a small set of images with data augmentation can detect germinal matrix hemorrhage on head ultrasounds with strong accuracy.




Similar content being viewed by others
References
Stoll BJ, Hansen NI, Bell EF et al (2015) Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 314:1039–1051
Plaisier A, Raets MMA, Ecury-Goossen GM et al (2015) Serial cranial ultrasonography or early MRI for detecting preterm brain injury? Arch Dis Child Fetal Neonatal Ed100:F293–300
Papile LA, Burstein J, Burstein R, Koffler H (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92:529–534
Li H, Parikh NA, Wang J et al (2019) Objective and automated detection of diffuse white matter abnormality in preterm infants using deep convolutional neural networks. Front Neurosci 13:610
LeCun Y, Bengio Y, Hinton G (2015) Deep learning. Nature 521:436–444
Kohli M, Prevedello LM, Filice RW, Geis JR (2017) Implementing machine learning in radiology practice and research. AJR Am J Roentgenol 208:754–760
Zernikow B, Holtmannspoetter K, Michel E et al (1998) Artificial neural network for risk assessment in preterm neonates. Arch Dis Child Fetal Neonatal Ed 79:F129-134
Ye H, Gao F, Yin Y et al (2019) Precise diagnosis of intracranial hemorrhage and subtypes using a three-dimensional joint convolutional and recurrent neural network. Eur Radiol 29:6191–6201
Hyun D, Brickson L (2016) Classification of neonatal brain ultrasound scans using deep convolutional neural networks. Stanford CS229:1–5
Altman DG, Machin D, Bryant TN, Gardner MJ (eds) (2000) Statistics with confidence, 2nd ed. BMJ Books. ISBN: 978–0–727–91375–3
Howard JP, Tan J, Shun-Shin MJ et al (2020) Improving ultrasound video classification: an evaluation of novel deep learning methods in echocardiography. J Med Artif Intell 3:4
Nguyen DT, Kang JK, Pham TD et al (2020) Ultrasound image-based diagnosis of malignant thyroid nodule using artificial intelligence. Sensors (Basel) 20:1822
Zhou LQ, Wang JY, Yu SY et al (2019) Artificial intelligence in medical imaging of the liver. World J Gastroenterol 25:672–682
Yi X, Adams S, Babyn P, Elnajmi A (2020) Automatic catheter and tube detection in pediatric x-ray images using a scale-recurrent network and synthetic data. J Digit Imaging 33:181–190
Ardila D, Kiraly AP, Bharadwaj S et al (2019) End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med 25:954–961
Majkowska A, Mittal S, Steiner DF et al (2020) Chest radiograph interpretation with deep learning models: assessment with radiologist-adjudicated reference standards and population-adjusted evaluation. Radiology 294:421–431
Zucker EJ, Barnes ZA, Lungren MP et al (2020) Deep learning to automate Brasfield chest radiographic scoring for cystic fibrosis. J Cyst Fibros 19:131–138
Simonyan K, Zisserman A (2015) Very deep convolutional networks for large-scale image recognition. arXiv:1409.1556
Yosinski J, Clune J, Bengio Y, Lipson H (2014) How transferable are features in deep neural networks? Proc. 27th International Conference on Neural Information Processing Systems 2:3320–3328
Parodi A, Morana G, Severino MS et al (2015) Low-grade intraventricular hemorrhage: is ultrasound good enough? J Matern Fetal Neonatal Med 28:2261–2264
Cizmeci MN, de Vries LS, Ly LG et al (2020) Periventricular hemorrhagic infarction in very preterm infants: characteristic sonographic findings and association with neurodevelopmental outcome at age 2 years. J Pediatr 217:79-85.e1
Kohli M, Geis R (2018) Ethics, artificial intelligence, and radiology. J Am Coll Radiol 15:1317–1319
Beam AL, Kohane IS (2018) Big data and machine learning in health care. JAMA 319:1317–1318
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, K.Y., Nowrangi, R., McGehee, A. et al. Assessment of germinal matrix hemorrhage on head ultrasound with deep learning algorithms. Pediatr Radiol 52, 533–538 (2022). https://doi.org/10.1007/s00247-021-05239-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-021-05239-w