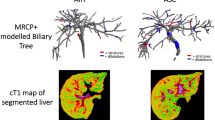
Abstract
Background
Primary sclerosing cholangitis, autoimmune hepatitis and autoimmune sclerosing cholangitis are forms of chronic, progressive autoimmune liver disease (AILD) that can affect the pediatric population.
Objective
To determine whether quantitative MRI- and laboratory-based biomarkers are associated with conventional imaging findings of portal hypertension (radiologic portal hypertension) in children and young adults with AILD.
Materials and methods
Forty-four patients with AILD enrolled in an institutional registry underwent a research abdominal MRI examination at 1.5 tesla (T). Five quantitative MRI techniques were performed: liver MR elastography, spleen MR elastography, liver iron-corrected T1 mapping, liver T2 mapping, and liver diffusion-weighted imaging (DWI, quantified as apparent diffusion coefficients). Two anatomical sequences were used to document splenomegaly, varices and ascites. We calculated aspartate aminotransferase (AST)-to-platelet ratio index (APRI) and fibrosis-4 (FIB-4) scores — laboratory-based biomarkers of liver fibrosis. We used receiver operating characteristic (ROC) curve analyses to establish the diagnostic performance of quantitative MRI and laboratory biomarkers for indicating the presence of radiologic portal hypertension.
Results
Twenty-three (52%) patients were male; mean age was 15.2±4.0 years. Thirteen (30%) patients had radiologic portal hypertension. Liver and spleen stiffness demonstrated the greatest diagnostic performance for indicating the presence of portal hypertension (area-under-the-ROC-curve [AUROC]=0.98 and 0.96, respectively). The APRI and FIB-4 scores also demonstrated good diagnostic performance (AUROC=0.87 and 0.88, respectively).
Conclusion
MRI-derived measures of liver and spleen stiffness as well as laboratory-based APRI and FIB-4 scores are highly associated with imaging findings of portal hypertension in children and young adults with AILD and thus might be useful for predicting portal hypertension impending onset and directing personalized patient management.




Similar content being viewed by others
References
Deneau M, Jensen MK, Holmen J et al (2013) Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: epidemiology and natural history. Hepatology 58:1392–1400
Singh H, Balouch F, Noble C, Lewindon P (2018) Evolving practice and changing phenotype in pediatric autoimmune liver disease: outcomes from an Australian center. J Pediatr Gastroenterol Nutr 67:80–85
Sun M, Kisseleva T (2015) Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol 39:S60–S63
Deneau MR, El-Matary W, Valentino PL et al (2017) The natural history of primary sclerosing cholangitis in 781 children: a multicenter, international collaboration. Hepatology 66:518–527
Bloom S, Kemp W, Lubel J (2015) Portal hypertension: pathophysiology, diagnosis and management. Intern Med J 45:16–26
Brancatelli G, Federle MP, Ambrosini R et al (2007) Cirrhosis: CT and MR imaging evaluation. Eur J Radiol 61:57–69
Trout AT, Sheridan RM, Serai SD et al (2018) Diagnostic performance of MR elastography for liver fibrosis in children and young adults with a spectrum of liver diseases. Radiology 2018:172099
Banerjee R, Pavlides M, Tunnicliffe EM et al (2014) Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol 60:69–77
Guimaraes AR, Siqueira L, Uppal R et al (2016) T2 relaxation time is related to liver fibrosis severity. Quant Imaging Med Surg 6:103–114
Koinuma M, Ohashi I, Hanafusa K, Shibuya H (2005) Apparent diffusion coefficient measurements with diffusion-weighted magnetic resonance imaging for evaluation of hepatic fibrosis. J Magn Reson Imaging 22:80–85
Yin M, Glaser KJ, Talwalkar JA et al (2016) Hepatic MR elastography: clinical performance in a series of 1,377 consecutive examinations. Radiology 278:114–124
Garteiser P, Doblas S, Van Beers BE (2018) Magnetic resonance elastography of liver and spleen: methods and applications. NMR Biomed 31:e3891
Nedredal GI, Yin M, McKenzie T et al (2011) Portal hypertension correlates with splenic stiffness as measured with MR elastography. J Magn Reson Imaging 34:79–87
Ronot M, Lambert S, Elkrief L et al (2014) Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol 24:1394–1402
Lin ZH, Xin YN, Dong QJ et al (2011) Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 53:726–736
Wai CT, Greenson JK, Fontana RJ et al (2003) A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38:518–526
Kim SY, Seok JY, Han SJ, Koh H (2010) Assessment of liver fibrosis and cirrhosis by aspartate aminotransferase-to-platelet ratio index in children with biliary atresia. J Pediatr Gastroenterol Nutr 51:198–202
Chou R, Wasson N (2013) Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med 158:807–820
Sterling RK, Lissen E, Clumeck N et al (2006) Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43:1317–1325
Messroghli DR, Radjenovic A, Kozerke S et al (2004) Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 52:141–146
Megremis SD, Vlachonikolis IG, Tsilimigaki AM (2004) Spleen length in childhood with US: normal values based on age, sex, and somatometric parameters. Radiology 231:129–134
Xanthakos SA, Podberesky DJ, Serai SD et al (2014) Use of magnetic resonance elastography to assess hepatic fibrosis in children with chronic liver disease. J Pediatr 164:186–188
Acknowledgments
This study was partially funded by: (1) a Cincinnati Children’s Hospital Medical Center (CCHMC) Academic and Research Committee grant and (2) the CCHMC Center for Autoimmune Liver Disease. Iron-corrected T1 image processing was performed by Perspectum Diagnostics (Oxford, UK) at no cost through a research agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Appendix 1: Imaging acquisition
Appendix 1: Imaging acquisition
Liver magnetic resonance elastography
Liver MR elastography was performed using an active-passive driver system (Resoundant Inc., Rochester, MN) operated at 60 Hz and a two-dimensional gradient recalled echo pulse sequence. The passive driver was placed over the right upper quadrant. Four axial slices positioned to cover the widest portion of the liver were acquired in four consecutive breath-holds at end-expiration. Four time points (phases) of the vibration cycle were collected for each slice. Two axial spatial saturation slabs were placed parallel to the imaging volume (i.e. in the S/I direction) to suppress the signal from flowing blood. Additional acquisition parameters were as follows: repetition time/echo time (TR/TE)=50/20 ms, flip angle=20°, field of view (FOV)=380 mm, matrix=252×80, section thickness=10 mm, slice gap=1 mm, acceleration=2, receiver bandwidth=288 Hz/pixel, and number of averages=1. Elastograms with 95% confidence maps were generated on the scanner based on MRI displacement data (four phase and four magnitude images per slice) using a direct inversion algorithm based on the Helmholtz equation [11].
Spleen magnetic resonance elastography
Spleen MR elastography was performed in a manner identical to liver MR elastography with the following exceptions: the passive driver was placed over the left upper quadrant, the four axial slices were positioned to cover the widest portion of the spleen, the FOV was 450 mm, and the matrix was 300×96.
Liver iron-corrected T1 mapping (cT1)
Liver iron-corrected T1 mapping was performed using a breath-hold modified Look-Locker inversion recovery technique (MOLLI) [20]. The acquisition was electrocardiographically (ECG)-triggered, with a pulse oximeter providing the cardiac synchronization signal. The following MOLLI acquisition scheme was used: 5-s (s) acquisition, 3-s pause, 3-s acquisition — which resulted in an 11-s breath-hold during which images at multiple time points along the T1 recovery curve were collected at a given slice location. The exact number of time points collected was dependent on the duration of the participant’s cardiac cycle. Four axial slices positioned to cover the widest portion of the liver were acquired in four consecutive 11-s breath-holds at end-expiration. Additional acquisition parameters were as follows: TR/TE=4.76/2.36 ms, flip angle=35°, FOV=440 mm, matrix=192×192, section thickness=8 mm, slice gap=7 mm, half Fourier=0.75, acceleration=2, receiver bandwidth=312 Hz/pixel, and number of averages=1. A multi-echo (n=8) gradient echo (TE=2.37–18.96 ms) acquisition was also performed to provide an estimate of T2*, which was used for the T1 iron correction. Mean whole-liver cT1 measurements were provided by Perspectum Diagnostics (Oxford, UK), which was blinded to all other imaging and clinical data.
Liver T2 mapping
Liver T2 mapping was performed using a respiratory-triggered multi-echo fast spin-echo technique and a total of 20 echo times (TE), ranging 12–240 ms. Four axial slices positioned to cover the widest portion of the liver were acquired. Additional parameters were as follows: TR=3,000 ms, FOV=360, matrix=256×179, slice thickness=8 mm, slice gap=8 mm, acceleration=2.2, receiver bandwidth=150 Hz/pixel, and number of averages=1. T2 maps were generated offline using MATLAB (MathWorks, Natick, MA).
Liver diffusion-weighted imaging (DWI)
Liver DWI was performed using a respiratory-triggered fat-suppressed single-shot echoplanar imaging pulse sequence with 5 b values (0 mm2/s, 100 mm2/s, 200 mm2/s, 500 mm2/s and 800 mm2/s). Twenty-seven axial slices positioned to cover the liver were acquired in three concatenations/packages. Four DWI images corresponding to the MR elastography anatomical levels were selected for analysis. Additional parameters were as follows: TR/TE=905/63.2 ms, FOV=400 mm, matrix=132×130, section thickness=6 mm, slice gap=0.6 mm, half Fourier=0.69, acceleration=2, receiver bandwidth=2,199 Hz/pixel, and number of averages=2. Apparent diffusion coefficient maps were generated by the scanner.
Rights and permissions
About this article
Cite this article
Dillman, J.R., Serai, S.D., Trout, A.T. et al. Diagnostic performance of quantitative magnetic resonance imaging biomarkers for predicting portal hypertension in children and young adults with autoimmune liver disease. Pediatr Radiol 49, 332–341 (2019). https://doi.org/10.1007/s00247-018-4319-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-018-4319-1