Abstract
Background
Human metapneumovirus (HMPV) was identified in 2001 and is a common cause of acute respiratory illness in young children. The radiologic characteristics of laboratory-confirmed HMPV acute respiratory illness in young children have not been systematically assessed.
Objective
We systematically evaluated the radiographic characteristics of acute respiratory illness associated with HMPV in a prospective cohort of pediatric patients.
Materials and methods
We included chest radiographs from children <5 years old with acute respiratory illness who were enrolled in the prospective New Vaccine Surveillance Network (NVSN) study from 2003 to 2009 and were diagnosed with HMPV by reverse transcription-polymerase chain reaction (RT-PCR). Of 215 HMPV-positive subjects enrolled at our tertiary care children’s hospital, 68 had chest radiographs obtained by the treating clinician that were available for review. Two fellowship-trained pediatric radiologists, independently and then in consensus, retrospectively evaluated these chest radiographs for their radiographic features.
Results
Parahilar opacities were the most commonly observed abnormality, occurring in 87% of children with HMPV. Hyperinflation also occurred frequently (69%). Atelectasis (40%) and consolidation (18%) appeared less frequently. Pleural effusion and pneumothorax were not seen on any radiographs.
Conclusion
The clinical presentations of HMPV include bronchiolitis, croup and pneumonia. Dominant chest radiographic abnormalities include parahilar opacities and hyperinflation, with occasional consolidation. Recognition of the imaging patterns seen with common viral illnesses like respiratory syncytial virus (RSV) and HMPV might facilitate diagnosis and limit unnecessary antibiotic treatment.
Similar content being viewed by others
Introduction
Human metapneumovirus (HMPV) was discovered in 2001 [1]. Human metapneumovirus is a member of the pneumovirus family of negative-sense, single-stranded ribonucleic acid (RNA) viruses that also includes respiratory syncytial virus (RSV). Human metapneumovirus has emerged as a significant contributor to upper and lower respiratory tract infections in infants and children and older adults, especially those with chronic medical conditions such as asthma, chronic obstructive pulmonary disease (COPD), and immune system compromise [2,3,4,5,6,7,8,9,10,11]. Human metapneumovirus is therefore a target for improved rapid diagnostic tests, as well as antiviral and vaccine development.
The burden of HMPV infection was evaluated in children in a prospective study undertaken by the Centers for Disease Control and Prevention (CDC) New Vaccine Surveillance Network (NVSN) from 2003 to 2009 [5]. Patients and healthy controls <5 years old were enrolled at three U.S. centers: Nashville, TN; Cincinnati, OH; and Rochester, NY. More than 10,000 children younger than 5 years were enrolled from outpatient clinics and emergency departments and as inpatients. The annual rate of hospitalization associated with HMPV was 1 per 1,000, similar to the rate for influenza virus and parainfluenza virus types 1–3 combined (1 per 1,000), but lower than the rate for RSV (3 per 1,000). Infants <6 months old with HMPV were more likely to be hospitalized than older children, and there was a high rate of outpatient visits associated with HMPV. Human metapneumovirus-associated visits to the outpatient clinic and emergency department continued until age 5 years [5]. It is important to note that HMPV is rarely detected in asymptomatic children [5, 6, 12, 13]. There are very rare reports of central nervous system disease associated with HMPV, but the biological mechanism of this is unclear [14, 15].
A subset of enrolled children identified as HMPV-positive at our institution in this prospective study had chest radiographs obtained as part of their routine clinical care. Thus we sought to describe the chest radiographic features of HMPV infection.
Materials and methods
The full design and methods of NVSN surveillance have been reported [5, 16,17,18,19]. Subjects included in the current study were enrolled in the winter and spring months from Nov. 1 through May 31 in the years 2003 through 2009. At our study site (Nashville), a total of 3,993 children younger than 5 years with acute respiratory illness or fever were enrolled. Hospitalized children were enrolled 4 days a week, outpatient surveillance was conducted 1–2 days per week, and subjects in the emergency department were enrolled 1–4 days per week. Acute respiratory illness was defined as an illness presenting with fever or one or more of the following symptoms: cough, earache, nasal congestion, rhinorrhea, sore throat, vomiting after coughing, wheezing, and labored, rapid or shallow breathing. Children were excluded if symptoms were present for >14 days, if they had chemotherapy-associated neutropenia, or if they had been hospitalized within the previous 4 days. Viral testing was performed in the research lab, and the results were not available to clinicians; thus clinical diagnoses were assigned by the patients’ health care providers independent of viral detection. A total of 215 subjects enrolled at our site tested positive for HMPV by reverse-transcriptase polymerase chain reaction (RT-PCR) [20]. We queried the NVSN database to identify subjects who had chest radiographs obtained; of these 215 HMPV-positive children, 77 (36%) underwent chest radiography at the discretion of the treating clinician. We retrieved the radiographic images for these subjects. Nine of the children had been enrolled at outlying hospitals in Nashville and the chest radiographs were unavailable. The remaining 68 HMPV-positive children with available chest radiographs made up our study population. The Vanderbilt University Institutional Review Board approved the study.
The subjects’ presenting chest radiographs were reviewed independently by two fellowship-trained pediatric radiologists (M.A.H. and S.P.S.), both with certificates of added qualification (CAQ) in pediatric radiology and more than 20 years of combined experience in pediatric radiology. The radiologists were blinded to the parameters and outcomes of the initial clinical study and were only told the patient population had tested positive for the virus. They systematically assessed the radiographs for a variety of common chest radiographic findings, including consolidation, parahilar opacities, peribronchial thickening, atelectasis, hypo-inflation, hyperinflation, pleural effusion, nodules and pneumothorax. Atelectasis was defined as present if there were signs of volume loss and if the opacity was not well defined and was present only on one view. Consolidation was defined as opacity visible on both anteroposterior and lateral views. There was no formal definition for interstitial disease, which was left at the discretion of the radiologist. Other abnormalities could be noted if present. These categories were decided a priori based on experience with radiologic assessment of bacterial and viral respiratory tract infections. The findings were entered into an electronic research database, REDCap [21], independently by each reviewer. Once the radiographs were independently reviewed, the radiologists performed a consensus review to discuss and resolve discrepancies.
Results
The 68 subjects had a median age of 15 months (mean 18 months, range 0–59 months). Forty-nine (72%) were inpatients, 18 (26%) were emergency department patients, and one was seen in the outpatient clinic. Thirty-six (53%) were male, with 21 (31%) white, 24 (35%) black, 13 (19%) Hispanic and 10 (15%) other race/ethnicity. Only four subjects had underlying medical conditions, which included chronic lung disease, developmental delay and congenital heart disease, genetic metabolic disease, and neurological disorder. The clinical diagnoses included pneumonia (n=34, 50%), asthma (n=25, 37%), bronchiolitis (n=14, 21%) and croup (n=1, 1%). Some children were assigned more than one diagnosis: 10 subjects were diagnosed with both asthma and pneumonia, and 2 children were diagnosed with both bronchiolitis and pneumonia. The mean age of children diagnosed with bronchiolitis was 10 months, while the mean age for both pneumonia and asthma was 20 months. No children were diagnosed with apnea or bacteremia. Of 49 children with complete records, 23 (47%) required supplemental oxygen, 7 (14%) were admitted to the intensive care unit and 5 (10%) required mechanical ventilation. There were no deaths.
The observed proportional agreement between the two radiologists’ independent interpretations was 96%. When considering overall radiologic findings, parahilar opacities were the most commonly observed abnormality, present in the majority of the patients (Table 1). Hyperinflation was another common finding, present in 69% of cases. Three of the children presented with hyperinflation only. Atelectasis and consolidation were observed less frequently but were not rare, at 40% and 18%, respectively. Pleural effusion and pneumothorax were not seen in any HMPV-infected child. Two of the subjects’ chest radiographs were normal.
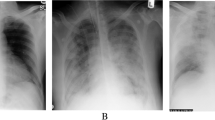
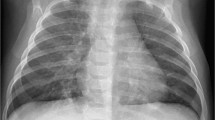
Subjects with different clinical diagnoses had different radiologic findings (Table 2). Children diagnosed with pneumonia were more likely to have consolidation and parahilar/peribronchial opacities, though consolidation was present only in 12 of 23. In contrast, children with asthma and bronchiolitis were more likely to have hyperinflation. Figures 1, 2, 3, and 4 demonstrate representative chest radiographic findings in HMPV-infected children with different clinical diagnoses and severity ranging from mild to severe disease, thus illustrating the wide spectrum of HMPV illness.
Radiography in a 19-month-old HMPV-infected previously healthy boy who presented with fever, upper respiratory tract symptoms and diarrhea. Physical exam revealed coarse breath sounds; complete blood count was normal. He was administered intravenous saline and discharged from the emergency department with the diagnoses of febrile illness and dehydration. a, b Anteroposterior (a) and lateral (b) chest radiographs show parahilar opacities. HMPV human metapneumovirus
Radiography in a 1-year-old HMPV-infected boy with history of occasional wheezing who presented with 5 days of fever, congestion and worsening cough. Physical exam revealed tachypnea, hypoxia, diffuse wheezes and bibasilar crackles. He was hospitalized with the diagnosis of pneumonia and treated with intravenous fluid, nebulized albuterol, supplemental oxygen and ceftriaxone. a, b Anteroposterior (a) and lateral (b) chest radiographs show parahilar opacities and hyperinflation. HMPV human metapneumovirus
Radiography in a 4-month-old HMPV-infected boy presenting with fever, cough and increased work of breathing. Physical exam revealed tachypnea and dyspnea; peripheral white blood count was 18.9 x 103/μL. He was hospitalized with the diagnosis of pneumonia and treated with ceftriaxone. Blood culture was negative. His anteroposterior portable chest radiograph (slightly rotated) showed hyperinflation, increased parahilar opacities and multiple areas of atelectasis. There was more confluent consolidation in the right upper lobe, a less frequent finding in children with HMPV. HMPV human metapneumovirus
Radiography in a 3-year-old boy with trisomy 21, atrioventricular septal defect, asthma and reflux. a Anteroposterior (AP) chest radiograph obtained at baseline 11 weeks prior to admission. The boy presented acutely with fever and dyspnea and required intubation and tested HMPV-positive. He was admitted to the intensive care unit and treated with intravenous antibiotics. Tracheal and blood cultures were negative. b Portable AP chest radiograph on admission shows multifocal consolidation and worsening parahilar opacities, a change from baseline. He experienced a prolonged hospitalization complicated by chylous left pleural effusion 3 weeks after admission. HMPV human metapneumovirus
Discussion
Radiography is commonly obtained as part of the clinical evaluation of children experiencing respiratory symptoms, primarily to exclude alternative diagnoses such as bacterial pneumonia and its complications, or foreign body aspiration. Recent guidelines for the management of community-acquired pneumonia in children recommend that chest radiographs be obtained only for outpatients with suspected or documented hypoxemia, significant respiratory distress or failed initial antibiotic therapy. Chest radiographs are recommended for all children hospitalized for pneumonia [22]. In contrast, chest radiographs are not recommended for the routine management of bronchiolitis [23]. Although chest radiograph findings might have influenced the clinical diagnosis for these children, the radiographs were ordered by clinicians with no knowledge of a viral pathogen.
Radiologists, particularly pediatric radiologists, are aware of the common viruses causing respiratory disease in children, namely RSV, influenza, parainfluenza and adenovirus. In the last decade human metapneumovirus has been recognized as a leading pathogen among viruses causing significant disease burden in children. As more rapid diagnostic tests for HMPV become available, radiologists should recognize this pathogen and its imaging findings. Our studies found parahilar opacities and hyperinflation to be the most prominent findings, but they also noted consolidation. Several reports have described some of the radiographic features of HMPV infection [24,25,26,27]; however most of these included fewer subjects, comprised retrospective convenience samples, or did not systematically evaluate radiographs. One report of 11 chest radiographs in children with radiologically diagnosed community-acquired pneumonia caused by HMPV described pulmonary infiltrates (alveolar or interstitial), hyperinflation and atelectasis [28]. Another report of chest CT findings in immunocompromised adults with HMPV reported asymmetrical pulmonary findings consisting of ground-glass opacity, airspace consolidation or bronchial wall thickening, in contrast to more symmetrical bilateral pulmonary infiltrates in immunocompromised adults with RSV-pneumonia [29].
The findings on chest radiographs of HMPV infection are similar to those associated with other common viral causes of lower respiratory tract infection, such as RSV, parainfluenza and influenza [30,31,32,33]. The radiographic findings of RSV infection and other viral respiratory tract infections have been described as parahilar peribronchial infiltrates and hyperexpansion [34]. These findings are not completely specific and are also noted in adenovirus, influenza and parainfluenza virus infections, although hyperexpansion was reported more commonly with RSV, and hilar adenopathy was seen more often with adenovirus [34]. Kern et al. [35] described central pneumonia and peribronchitis as common chest radiographic findings in young children with RSV infection. Because terms placed in a radiographic report are not uniform and are interpreted by clinicians in different ways, the terms “central pneumonia” and “parahilar infiltrates” are likely describing the same findings [36]. Pleural effusions and lobar pneumonia were reported to be uncommon with RSV infection, similar to our findings for HMPV infection. One study reported frequent hilar adenopathy associated with RSV infection [37], but we did not observe this in any child with HMPV infection. Some of the findings we observed could be caused by chronic lung disease; however only 4 children out of 68 had underlying medical conditions.
Although radiologists should recognize these viral infection patterns on chest radiographs, they cannot definitively identify the virus without laboratory confirmation. Similarly, the clinical signs and symptoms do not reliably discriminate among these pathogens [13]. It is important to identify parahilar opacities and hyperinflation consistent with a viral pattern of disease because the child can be treated supportively without antibiotics in the absence of a confirmed superimposed bacterial pneumonia.
Our study has some limitations. We present a retrospective review of radiographs in children with HMPV infection from only one of the three initial study sites. Furthermore, not all children who tested positive for HMPV underwent chest radiography and those who did undergo chest radiography might have had more severe symptoms. Moreover, the clinical diagnoses were assigned with knowledge of the initial chest radiograph reading, which likely influenced the choice of diagnosis. In addition, upon review of the chest radiographs, the study radiologists were aware of the diagnosis, and perhaps were biased toward finding abnormalities.
Conclusion
Human metapneumovirus is a leading cause of acute lower respiratory infection in children and adults, with a substantial disease burden similar in degree to that of influenza virus [5]. There are no vaccines or antiviral drugs available against HMPV. Radiologists should be aware of this new pathogen, and to that end this study serves to document radiographic findings in young children with HMPV infection, most commonly parahilar opacities and hyperinflation without effusions or lobar consolidation.
References
van den Hoogen BG, de Jong JC, Groen J et al (2001) A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med 7:719–724
Boivin G, De Serres G, Cote S et al (2003) Human metapneumovirus infections in hospitalized children. Emerg Infect Dis 9:634–640
Esper F, Martinello RA, Boucher D et al (2004) A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis 189:1388–1396
Williams JV, Harris PA, Tollefson SJ et al (2004) Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 350:443–450
Edwards KM, Zhu Y, Griffin MR et al (2013) Burden of human metapneumovirus infection in young children. N Engl J Med 368:633–643
Jain S, Williams DJ, Arnold SR et al (2015) Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 372:835–845
Martinello RA, Esper F, Weibel C et al (2006) Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J Inf Secur 53:248–254
Walsh EE, Peterson DR, Falsey AR (2008) Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med 168:2489–2496
Shahda S, Carlos WG, Kiel PJ et al (2011) The human metapneumovirus: a case series and review of the literature. Transpl Infect Dis 13:324–328
Chu HY, Renaud C, Ficken E et al (2014) Respiratory tract infections due to human metapneumovirus in immunocompromised children. J Pediatric Infect Dis Soc 3:286–293
Widmer K, Zhu Y, Williams JV et al (2012) Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis 206:56–62
Self WH, Williams DJ, Zhu Y et al (2016) Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis 213:584–591
Williams JV, Wang CK, Yang CF et al (2006) The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis 193:387–395
Schildgen O, Glatzel T, Geikowski T et al (2005) Human metapneumovirus RNA in encephalitis patient. Emerg Infect Dis 11:467–470
Sanchez Fernandez I, Rebollo Polo M, Munoz-Almagro C et al (2012) Human metapneumovirus in the cerebrospinal fluid of a patient with acute encephalitis. Arch Neurol 69:649–652
Griffin MR, Walker FJ, Iwane MK et al (2004) Epidemiology of respiratory infections in young children: insights from the new vaccine surveillance network. Pediatr Infect Dis J 23:S188–S192
Iwane MK, Edwards KM, Szilagyi PG et al (2004) Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics 113:1758–1764
Poehling KA, Edwards KM, Weinberg GA et al (2006) The underrecognized burden of influenza in young children. N Engl J Med 355:31–40
Hall CB, Weinberg GA, Iwane MK et al (2009) The burden of respiratory syncytial virus infection in young children. N Engl J Med 360:588–598
Klemenc J, Asad Ali S, Johnson M et al (2012) Real-time reverse transcriptase PCR assay for improved detection of human metapneumovirus. J Clin Virol 54:371–375
Harris PA, Taylor R, Thielke R et al (2009) Research electronic data capture (REDCap) — a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381
Bradley JS, Byington CL, Shah SS et al (2011) Executive summary: the management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 53:617–630
Ralston SL, Lieberthal AS, Meissner HC et al (2014) Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 134:e1474–e1502
Akhras N, Weinberg JB, Newton D (2010) Human metapneumovirus and respiratory syncytial virus: subtle differences but comparable severity. Infect Dis Rep 2:e12
Martin ET, Kuypers J, Heugel J et al (2008) Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis 62:382–388
Morrow BM, Hatherill M, Smuts HE et al (2006) Clinical course of hospitalised children infected with human metapneumovirus and respiratory syncytial virus. J Paediatr Child Health 42:174–178
Pitoiset C, Darniot M, Huet F et al (2010) Human metapneumovirus genotypes and severity of disease in young children (n=100) during a 7-year study in Dijon hospital, France. J Med Virol 82:1782–1789
Nascimento-Carvalho CM, Cardoso MR, Ruuskanen O et al (2011) Sole infection by human metapneumovirus among children with radiographically diagnosed community-acquired pneumonia in a tropical region. Influenza Other Respir Viruses 5:285–287
Syha R, Beck R, Hetzel J et al (2012) Human metapneumovirus (HMPV) associated pulmonary infections in immunocompromised adults — initial CT findings, disease course and comparison to respiratory-syncytial-virus (RSV) induced pulmonary infections. Eur J Radiol 81:4173–4178
Swingler GH (2000) Radiologic differentiation between bacterial and viral lower respiratory infection in children: a systematic literature review. Clin Pediatr 39:627–633
Simpson W, Hacking PM, Court SD et al (1974) The radiological findings in respiratory syncytial virus infection in children. II. The correlation of radiological categories with clinical and virological findings. Pediatr Radiol 2:155–160
Simpson W, Hacking PM, Court SD et al (1974) The radiological findings in respiratory syncytial virus infection in children. Part I. Definitions and interobserver variation in the assessment of abnormalities on the chest X-ray. Pediatr Radiol 2:97–100
Rice RP, Loda F (1966) A roentgenographic analysis of respiratory syncytial virus pneumonia in infants. Radiology 87:1021–1027
Wildin SR, Chonmaitree T, Swischuk LE (1988) Roentgenographic features of common pediatric viral respiratory tract infections. Am J Dis Child 142:43–46
Kern S, Uhl M, Berner R et al (2001) Respiratory syncytial virus infection of the lower respiratory tract: radiological findings in 108 children. Eur Radiol 11:2581–2584
Spottswood SE, Liaw K, Hernanz-Schulman M et al (2009) The clinical impact of the radiology report in wheezing and nonwheezing febrile children: a survey of clinicians. Pediatr Radiol 39:348–353
Odita JC, Nwankwo M, Aghahowa JE (1989) Hilar enlargement in respiratory syncytial virus pneumonia. Eur J Radiol 9:155–157
Acknowledgments
This work was supported by grants from the Centers for Disease Control and Prevention (CDC) U38 CCU417958 and U01 IP000022 (K.M.E., M.R.G.) and the National Institutes of Health (NIH) AI-085062 (J.V.W.). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
J.V. Williams is on the Scientific Advisory Board for Quidel, Inc., and the Independent Data Monitoring Committee for GlaxoSmithKline. M.A. Hilmes, F.D. Dunnavant, S.P. Singh, W.D. Ellis, D.C. Payne, Y. Zhu, M.R. Griffin and K.M. Edwards have no conflicts to disclose.
Rights and permissions
About this article
Cite this article
Hilmes, M.A., Daniel Dunnavant, F., Singh, S.P. et al. Chest radiographic features of human metapneumovirus infection in pediatric patients. Pediatr Radiol 47, 1745–1750 (2017). https://doi.org/10.1007/s00247-017-3943-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-017-3943-5