Abstract
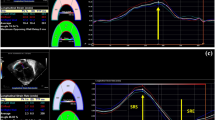
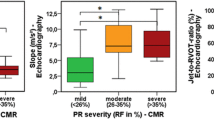
Assessment of pulmonary regurgitation (PR) guides treatment for patients with congenital heart disease. Quantitative assessment of PR fraction (PRF) by echocardiography is limited. Cardiac MRI (cMRI) is the reference-standard for PRF quantification. We created an algorithm to predict cMRI-quantified PRF from echocardiography using machine learning (ML). We retrospectively performed echocardiographic measurements paired to cMRI within 3 months in patients with ≥ mild PR from 2009 to 2022. Model inputs were vena contracta ratio, PR index, PR pressure half-time, main and branch pulmonary artery diastolic flow reversal (BPAFR), and transannular patch repair. A gradient boosted trees ML algorithm was trained using k-fold cross-validation to predict cMRI PRF by phase contrast imaging as a continuous number and at > mild (PRF ≥ 20%) and severe (PRF ≥ 40%) thresholds. Regression performance was evaluated with mean absolute error (MAE), and at clinical thresholds with area-under-the-receiver-operating-characteristic curve (AUROC). Prediction accuracy was compared to historical clinician accuracy. We externally validated prior reported studies for comparison. We included 243 subjects (median age 21 years, 58% repaired tetralogy of Fallot). The regression MAE = 7.0%. For prediction of > mild PR, AUROC = 0.96, but BPAFR alone outperformed the ML model (sensitivity 94%, specificity 97%). The ML model detection of severe PR had AUROC = 0.86, but in the subgroup with BPAFR, performance dropped (AUROC = 0.73). Accuracy between clinicians and the ML model was similar (70% vs. 69%). There was decrement in performance of prior reported algorithms on external validation in our dataset. A novel ML model for echocardiographic quantification of PRF outperforms prior studies and has comparable overall accuracy to clinicians. BPAFR is an excellent marker for > mild PRF, and has moderate capacity to detect severe PR, but more work is required to distinguish moderate from severe PR. Poor external validation of prior works highlights reproducibility challenges.





Similar content being viewed by others
Abbreviations
- AUROC:
-
Area under the receiver operating characteristic curve
- BPA:
-
Branch pulmonary artery
- BPAFR:
-
Branch pulmonary artery flow reversal
- cMRI:
-
Cardiac magnetic resonance imaging
- ICC:
-
Intraclass correlation coefficient
- MAE:
-
Mean absolute error
- ML:
-
Machine learning
- MPA:
-
Main pulmonary artery
- OLS:
-
Ordinary least squares
- PHT:
-
Pressure halftime
- PR:
-
Pulmonary regurgitation
- PRF:
-
Pulmonary regurgitation fraction
- TAP:
-
Transannular patch
- VCR:
-
Vena contracta ratio
References
Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M (2018) AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. https://doi.org/10.1161/CIR.0000000000000602
Geva T (2011) Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson 13:1–24
Rebergen SA, van der Wall EE, Doornbos J, de Roos A (1993) Magnetic resonance measurement of velocity and flow: technique, validation, and cardiovascular applications. Am Heart J 126:1439–1456
Redington AN (2006) Determinants and assessment of pulmonary regurgitation in tetralogy of Fallot: practice and pitfalls. Cardiol Clin 24:631–639
Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, van Rossum AC, Shaw LJ, Yucel EK (2004) Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J 25:1940–1965
Bonello B, Kilner PJ (2012) Review of the role of cardiovascular magnetic resonance in congenital heart disease, with a focus on right ventricle assessment. Arch Cardiovasc Dis 105:605–613
Marwick TH, Neubauer S, Petersen SE (2013) Use of cardiac magnetic resonance and echocardiography in population-based studies: why, where, and when? Circ: Cardiovasc Imaging 6:590–596
Bansal N, Gupta P, Joshi A, Zerin JM, Aggarwal S (2019) Utility of Doppler echocardiography to estimate the severity of pulmonary valve regurgitation fraction in patients with repaired tetralogy of Fallot. Pediatr Cardiol 40:404–411
Beurskens NE, Gorter TM, Pieper PG, Hoendermis ES, Bartelds B, Ebels T, Berger RM, Willems TP, van Melle JP (2017) Diagnostic value of Doppler echocardiography for identifying hemodynamic significant pulmonary valve regurgitation in tetralogy of Fallot: comparison with cardiac MRI. Int J Cardiovasc Imaging 33:1723–1730
Li W, Davlouros PA, Kilner PJ, Pennell DJ, Gibson D, Henein MY, Gatzoulis MA (2004) Doppler-echocardiographic assessment of pulmonary regurgitation in adults with repaired tetralogy of Fallot: comparison with cardiovascular magnetic resonance imaging. Am Heart J 147:165–172
Mercer-Rosa L, Yang W, Kutty S, Rychik J, Fogel M, Goldmuntz E (2012) Quantifying pulmonary regurgitation and right ventricular function in surgically repaired tetralogy of Fallot: a comparative analysis of echocardiography and magnetic resonance imaging. Circ: Cardiovasc Imaging 5:637–643
Puchalski MD, Askovich B, Sower CT, Williams RV, Minich LL, Tani LY (2008) Pulmonary regurgitation: determining severity by echocardiography and magnetic resonance imaging. Congenit Heart Dis 3:168–175
Silversides CK, Veldtman GR, Crossin J, Merchant N, Webb GD, McCrindle BW, Siu SC, Therrien J (2003) Pressure half-time predicts hemodynamically significant pulmonary regurgitation in adult patients with repaired tetralogy of Fallot. J Am Soc Echocardiogr 16:1057–1062
Renella P, Aboulhosn J, Lohan DG, Jonnala P, Finn JP, Satou GM, Williams RJ, Child JS (2010) Two-dimensional and Doppler echocardiography reliably predict severe pulmonary regurgitation as quantified by cardiac magnetic resonance. J Am Soc Echocardiogr 23:880–886
Deo RC (2015) Machine learning in medicine. Circulation 132:1920–1930
Jone P-N, Gearhart A, Lei H, Xing F, Nahar J, Lopez-Jimenez F, Diller G-P, Marelli A, Wilson L, Saidi A (2022) Artificial intelligence in congenital heart disease: current state and prospects. JACC: Adv 1:100153
Gearhart A, Gaffar S, Chang AC (2020) A primer on artificial intelligence for the paediatric cardiologist. Cardiol Young 30:934–945
Chen T, Guestrin C (2016) XGBoost: a scalable tree boosting system. Paper/Poster presented 2016/08/13/, https://arxiv.org/abs/1603.02754. Accessed 04 June 2023
Scikit-Learn Developers. Iterative imputer. https://scikit-learn.org/stable/modules/generated/sklearn.impute.IterativeImputer.html. Accessed 04 June 2023
Dai W, Nazzari H, Namasivayam M, Hung J, Stultz CM (2023) Identifying aortic stenosis with a single parasternal long-axis video using deep learning. J Am Soc Echocardiogr 36:116–118. https://doi.org/10.1016/j.echo.2022.10.014
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conception and design of the study:JC, SQD, NA, GNN, DE; Acquisition of data: JC, SQD, DMB, SB, RC, MG, KH, MK, GK, XM, MM, EAP, MT, JLY, DE; Analysis and interpretation of data: JC, SQD, NA, GNN, DE; Drafting the article or revising it critically for important intellectual content: JC, SQD, NA, DMB, SB, RC, MG, KH, MK, GK, XM, MM, EAP, MT, JLY, GNN, DE; final approval of the version to be submitted: All authors have approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
David Ezon is CEO of Tapestry Insight, Inc. Girish Nadkarni reports consultancy agreements with AstraZeneca, BioVie, GLG Consulting, Pensieve Health, Reata, Renalytix, Siemens Healthineers and Variant Bio; research funding from Goldfinch Bio and Renalytix; honoraria from AstraZeneca, BioVie, Lexicon, Daiichi Sankyo, Menarini Health and Reata; patents or royalties with Renalytix; owns equity and stock options in Pensieve Health and Renalytix as a scientific cofounder; owns equity in Verici Dx; has received financial compensation as a scientific board member and advisor to Renalytix; serves on the advisory board of Neurona Health; and serves in an advisory or leadership role for Pensieve Health and Renalytix. No other authors report competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cohen, J., Duong, S.Q., Arivazhagan, N. et al. Machine Learning Quantification of Pulmonary Regurgitation Fraction from Echocardiography. Pediatr Cardiol (2024). https://doi.org/10.1007/s00246-024-03511-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00246-024-03511-y