Abstract
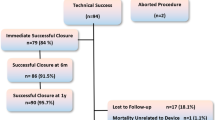
The experience with percutaneous closure of postoperative residual ventricular septal defects (VSDs) is expanding with improved device technology and techniques. To report our experience with percutaneous closure of residual VSDs after cardiac surgeries. Retrospective clinical data review of patients who had percutaneous closure of postoperative residual VSDs at our institution between 2010 and 2022. Patients’ demographics, procedural, and follow-up data were looked at. Twelve patients (50% males) with a median age of 9.2 years (range 0.9–22) were identified. Baseline surgeries were 8 tetralogy of Fallot corrections, 2 pulmonary bandings for large muscular VSD (mVSD) including 1 coarctation repair, 1 atrioventricular septal defect repair, 1 sub-aortic membrane resection-induced iatrogenic VSD, 1 isolated VSD closure, and 1 additional mVSD. Median duration between baseline surgery and percutaneous VSD closure was 2.2 years (range 0.2–8.3). Residual VSD shunting was secondary to surgical patch leakage in 8/12 patients. The median angiographic defect diameter was 6.8 mm (range 4.8–14). The defect was balloon-calibrated in 3/12 patients. Defects were tackled retrogradely in 3/9 patients. Amplatzer Membranous VSD occluder (n = 1), Lifetech Multifunctional (n = 5), Membranous (n = 1) and muscular VSD occluders (n = 2) and Occlutech Membranous (n = 1) and Muscular (n = 2) VSD occluders were used. The procedure was successful in 10/12 patients. Two devices embolized to the pulmonary artery and were snare-retrieved. Both patients were referred for surgery. The median follow-up was 1.3 years (range 0.1–12). Six-month ultrasound showed one trivial residual shunt and one mild right ventricular outflow obstruction. One patient is receiving targeted therapy for pulmonary hypertension at 2 years of follow-up. Transcatheter closure of postoperative residual VSDs is a feasible yet challenging intervention. Procedural complications can be encountered.





Similar content being viewed by others
References
Deng X, Huang P, Luo J et al (2020) Residual shunts following isolated surgical ventricular septal defect closure: risk factors and spontaneous closure. Pediatr Cardiol 41(1):38–45
Baspinar O, Kilinc M, Kervancioglu M, Irdem A (2011) Transcatheter closure of a residual patent ductus arteriosus after surgical ligation in children. Korean Circ J 41(11):654–657
Bibevski S, Ruzmetov M, Mendoza L et al (2020) The destiny of postoperative residual ventricular septal defects after surgical repair in infants and children. World J Pediatr Congenit Heart Surg 11(4):438–443
Kouakou NYN, Song J, Huh J, Kang IS (2019) The experience of transcatheter closure of postoperative ventricular septal defect after total correction. J Cardiothorac Surg 14(1):104
Walsh MA, Coleman DM, Oslizlok P, Walsh KP (2006) Percutaneous closure of postoperative ventricular septal defects with the Amplatzer device. Catheter Cardiovasc Interv 67(3):445–451
Ergün S, Genç SB, Yıldız O et al (2020) Predictors of a complicated course after surgical repair of tetralogy of Fallot. Turk Gogus Kalp Damar Cerrahisi Derg 28(2):264–273
Yin S, Zhu D, Lin K, An Q (2014) Perventricular device closure of congenital ventricular septal defects. J Cardiac Surg 29:390–400
Bergmann M, Germann CP, Nordmeyer J, Peters B, Berger F, Schubert S (2021) Short- and long-term outcome after interventional vsd closure: a single-center experience in pediatric and adult patients. Pediatr Cardiol 42(1):78–88
Nagi Haddad R, Ly R, Iserin L, Malekzadeh-Milani S (2021) Aorta-to-right ventricle neoshunt closure using an Amplatzer Duct Occluder II device. J Card Surg 36(6):2156–2159
Gu X, Zhang Q, Sun H, Fei J, Zhang X, Kutryk MJ (2017) Transcatheter closure versus repeat surgery for the treatment of postoperative left-to-right shunts: a single center 15-year experience. Cardiol Res 8(6):286–292
Taha FA, Alnozha F, Amoudi O, Almutairi M, Abuelatta R (2022) Transcatheter closure of residual and iatrogenic ventricular septal defects: tertiary center experience and outcome. Pediatr Cardiol 43(2):308–323
Haddad RN, Daou LS, Saliba ZS (2020) Percutaneous closure of restrictive-type perimembranous ventricular septal defect using the new KONAR multifunctional occluder: midterm outcomes of the first middle-eastern experience. Catheter Cardiovasc Interv. https://doi.org/10.1002/ccd.28678
Yang L, Tai BC, Khin LW, Quek SC (2014) A systematic review on the efficacy and safety of transcatheter device closure of ventricular septal defects (VSD). J Interv Cardiol 27(3):260–272
Knauth AL, Lock JE, Perry SB et al (2004) Transcatheter device closure of congenital and postoperative residual ventricular septal defects. Circulation 110(5):501–507
Gu MB, Bai Y, Zhao XX, Zheng X, Li WP, Qin YW (2009) Transcatheter closure of postoperative residual perimembranous ventricular septal defects. Ann Thorac Surg 88(5):1551–1555
Zhou W, Li F, Fu L et al (2016) Clinical experience of transcatheter closure for residual ventricular septal defect in pediatric patients. Congenit Heart Dis 11(4):323–331
Shahanavaz S, Winlaw DS, Opotowsky AR (2022) What is blocking transcatheter ventricular septal defect closure? J Am Heart Assoc 11(7):e024963
Morgan GJ (2016) Occlutech® muscular ventricular septal defect device: the first reported human use. Cardiol Young 26(7):1448–1451
Atik SU, Saltik L (2018) Transcatheter closure of ventricular septal defect with two different devices. Cardiol Young 28(11):1364–1366
Sadiq M, Qureshi AU, Younas M, Arshad S, Hyder SN (2022) Percutaneous closure of ventricular septal defect using LifeTech™ Konar-MF VSD Occluder: initial and short-term multi-institutional results. Cardiol Young 32(5):755–761
Haddad RN, Daou L, Saliba Z (2019) Device closure of perimembranous ventricular septal defect: choosing between Amplatzer occluders. Front Pediatr 16(7):300
Tanidir IC, Baspinar O, Saygi M, Kervancioglu M, Guzeltas A, Odemis E (2020) Use of Lifetech™ Konar-MF, a device for both perimembranous and muscular ventricular septal defects: a multicentre study. Int J Cardiol 1(310):43–50
Kilicgedik A, Karabay CY, Aung SM et al (2012) A successful percutaneous closure of ventricular septal defect following septal myectomy in patients with hypertrophic obstructive cardiomyopathy. Perfusion 27:253–255
Parsons C, Zhao CB, Huang J (2020) Closure of an iatrogenic ventricular septal defect using a hybrid approach and echocardiographic guidance. Ann Card Anaesth 23(2):212–215
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Osman Baspinar, Pelin Kosger and Derya Aydin Sahin. The first draft of the manuscript was written by Pelin Kosger and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baspinar, O., Kosger, P. & Aydin Sahin, D. Percutaneous Closure of Hemodynamically Significant Postoperative Residual Ventricular Septal Defects. Pediatr Cardiol 45, 272–281 (2024). https://doi.org/10.1007/s00246-023-03366-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-023-03366-9