Abstract
Implantable cardiac monitors (ICM) allow for symptom–rhythm correlation. Current manufacturer recommendations call for implantation of ICMs diagonally in the left anterior chest. Complications such as skin tenting and device erosion have occurred using this technique in pediatric patients. The purpose of this study was to assess the safety and efficacy of implanting ICMs via new vertical–parasternal technique (VP) compared to manufacturer-recommended diagonal technique (D) in pediatric patients. Single-center, IRB-approved retrospective study of pediatric patients that underwent ICM implantation from 01/01/2017 to 12/01/2021. All implants were performed after informed consent, under sterile conditions in the electrophysiology laboratory. Data collected included demographics, implant orientation (VP or D), complications, device type, presence of P-wave, and measurement of R-wave amplitude at implantation and follow-up. ICMs were implanted in 34 patients without congenital heart disease. Initial R-wave amplitude average for VP 1.00, D 0.99 (p = NS). Follow-up R-wave amplitude was 0.97 VP and 0.93 for D (p = NS). Median follow-up period for VP was 11 and for D was 20 months (p = NS). D cohort had only post-procedural complication due to skin tenting of the ICM in child < 2.5 years of age. No skin tenting, erosions, or complications occurred in the vertical–parasternal implant technique. Vertical–Parasternal ICM implantation is as safe and effective as the manufacturer-recommended diagonal implant. Short- and long-term data demonstrate an equivalent R-wave detection and no significant signal deterioration, even in very young children. No skin tenting, erosions, or complications occurred in the vertical parasternal implant technique.
Similar content being viewed by others
Background
Implantable cardiac monitors (ICMs) are subcutaneously inserted devices that provide continuous, single-lead electrocardiograms for long-term evaluation of the patient’s cardiac rhythm. ICMs are helpful in correlating the patient’s cardiac rhythm with infrequent, concerning symptoms. Using simple external heart rhythm monitoring systems risks not capturing a dangerous underlying arrhythmia during rare events [1]. Recent studies have demonstrated “silent” arrhythmias were recorded in as many as 71% of patients using ICM monitoring [2, 3].
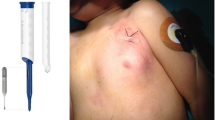
In 1995, Circulation first published support for ICM use in adults. The devices used in that study were 25.4 cubic cm and were too large for pediatric implant [4]. In 2002, the first case series of ICM use in pediatric patients demonstrated both safety and efficacy [5]. Over the last two decades, ICMs have become significantly smaller, measuring as small as 1.2 cm3, with improved functionality. Modern miniaturized devices now report findings via Bluetooth®, paired with a cell phone, in real-time, instead of manual in-home or office transmission [6, 7]. In spite of the improvements in size, case reports of both skin tenting and device erosion have been published in children and adolescents [8, 9] (Image 1). Currently, device manufacturer’s recommend diagonal (D) implantation of ICM over the left anterior chest at the 4th intercostal space (Image 2). As improvements have been made in device size and functionality, device implantation technique should also be assessed for improvement.
Objective
The purpose of this study was to assess the safety and efficacy of implanting ICMs with novel vertical–parasternal (VP) technique, compared with manufacturer-recommended diagonal (D) technique in a pediatric population.
Methods
This was a single-center study performed at a large, quaternary academic center. The study was an IRB-approved, retrospective chart review of data collected from patients in the pediatric electrophysiology lab. All procedures were performed after informed consent was obtained from patients and/or families. Procedures were performed under sterile conditions in the electrophysiology laboratory. All patients received deep sedation with cardiac anesthesiology and, per institutional protocol, received weight-based dose of cefazolin or clindamycin if allergic, prior to device insertion. Devices used in this study include Abbott Confirm Rx (Chicago, IL) and Medtronic LINQ II (Ireland). Both the Abbott Confirm RX and LINQ II have a volume of 1.4 cubic cm.
Manufacturer recommendations were followed for D implantation, beginning at the left sternal wall at the 4th intercostal space with diagonal insertion across the left anterior chest wall (Image 2). VP implantation was performed by novel technique with incision 1 cm to left and parallel to the sternum at the 3–4th intercostal space. Devices were inserted in a vertical position running parallel to the sternum in the left anterior chest (Images 3, 4). Patients were excluded from enrollment if they were over 21 years old or had previously diagnosed congenital heart disease.
R-wave detection was assessed by the ICM device programmer using a pre-programmed algorithm. R-waves were manually confirmed by pediatric electrophysiologist at time of implant. Follow-up visits were often performed virtually. In-office follow-up did not occur at set intervals for all patients due to the limiting of in-office visits during the COVID pandemic. In-office visits occurred, on average, once every six months. At both in-office and virtual follow-up, devices were interrogated and patients were assessed for complications. Given inconsistent in-office follow-up, virtual transmissions which were also performed the evening prior to in-office visits and used as the standard when evaluating signal deterioration after implantation. Skin tenting was defined as significant elevation of the skin, along the breadth of the device, with the patient in a supine position. Skin tenting was documented when present; however, skin tenting in isolation was not considered a complication. Patients performed transfer of ICM recordings once a month or sooner in case of patient-activated event or reported symptom.
Statistical analysis was performed based on ICM implantation direction (VP or D), BSA, and age. ANOVA analysis was performed to compare average R-wave amplitude at the time of implantation and via patient transmissions. Significance value for all tests was set at 0.05.
Results
A total of 34 ICMs were implanted in patients meeting enrollment criteria. The mean age at implantation for all participants was 12.04 years (range 1–21), 12.5 years for VP, and 11.5 years for D cohorts (P = NS) (Table 1). Overall 53% of the patients were male, with VP 57% male and D 46% male. Average patient BSA for all participants at time of implantation was 1.26 m2 (range 0.45–1.94). VP cohort had an average BSA of 1.22 m2 and 1.29 m2 for D cohort. Indication for implantation was similar for all groups with syncope and palpitations being the most common. The median follow period was 14 months (range 4–24) for all patients, 11 months for VP, and 18 months for D cohorts, respectively (Fig. 1).
P-waves were visually observed in all cases regardless of implant technique. The average R-wave at the time of implant was 1.00 mV for VP and 0.99 mV for D. Average R-wave at follow-up checks measured 0.97 mV, SD 0.39 for VP and 0.93 mV, SD 0.24 for D. There was no significant difference between either cohort in terms of initial detection nor signal degradation over time. Linear regression analysis did not show a significant correlation between either age, BSA, or R-wave amplitude (p = NS).
A total of 13 patients had a BSA less than 1.0, 7 VP and 6 D patients. R-wave amplitude was 1.12 mV among this subset, compared to 0.88 mV for BSA > 1. During follow-up, there were no significant changes in R-wave amplitude for either subset, 1.03 mV for BSA < 1 and 0.86 mV for BSA > 1 (P = NS for all).
Looking specifically at infants and children, a total of 6 patients were less than 5 years of age at the time of the implant, 3 each in VP and D cohorts. R-wave amplitude at implant was 1.2 mV in both cohorts. The average initial R-wave amplitude was 1.03 mV and showed no decrease during follow-up for these patients. Skin tenting was noted in 3 patients in the D cohort at both implantation and follow-up. Of note, a 2.5-year-old patient from the D cohort had skin tenting and peri-procedural infection of the ICM pocket, so the device was prematurely explanted. No patients in the VP cohort had skin tenting at the time of device implantation or follow-up visits. A total of three patients within the VP cohort were < 3 years old at the time of implantation. No patients from the VP cohort experienced complications. The VP group had no skin tenting seen at either implant or follow-up evaluation.
Discussion
This study aimed to evaluate the novel VP technique for safety and efficacy when compared with manufacturer-recommended D technique.
Our results demonstrate no significant difference in signal detection at the time of implant and over the life of the device between VP and D techniques. R-wave amplitudes detected in both VP and D implantation were excellent. Bluetooth® ICM transmission allowed for consistent follow-up and zero patients lost to follow-up, even as clinic visits were limited during the coronavirus pandemic.
Multiple reports of skin tenting and device erosion following ICM implantation have been published. A majority of these cases involved young children with diagonal implanted ICMs. Initially in 2016, Chaouki et al. reported a case of device migration with subsequent erosion in a 6-year-old boy after the device was inserted via D technique at a 45 degree angle [5]. Then, in 2020, Zakhar et al. found that 4 of 81 patients (4.7%) receiving ICM implantation at a diagonal, 45 degree angle experienced device erosion [6]. Notably, two of these patients were 8 years old at the time of implant and had thin-body habitus. A study from Bezzerides et al. in 2019 found an overall rate of complications of 4.5% in 133 patients over a 12-month follow-up period. However, the study noted a much higher rate of complications, 27%, among patients less than 5 years old. This study used the LINQ Reveal device (volume 1.2 cc, length 4.48 cm) [10]. Both devices used in our study, LINQ II (1.4 cc, 4.5 cm) and Confirm RX (1.4 cc, 4.9 cm), measure slightly larger. A recent study from Italy, 2022, evaluating ICM performance over a 10-year period had a cohort of 33 patients between 1 and 5 years old. Within this cohort, one case of device erosion occurred in a 3-year-old child [11].
Based on these data, young patients with thin-body habitus appear to be most vulnerable to skin tenting which can progress to device migration and eventual erosion. We developed the novel VP technique in response to these data and a patient in our institution who experienced skin tenting and peri-procedural infection at the time of implantation in 2018.
Other non-manufacturer-recommended ICM implantation techniques have been used at other centers. The 2019 Bezzarides study from Boston Children’s Hospital used axillary ICM implantation in 31% of the cohort. However, they did demonstrate that axillary-implanted ICMs had significantly lower R-wave amplitudes than their manufacturer-recommended D implantation cohort [10].
In our study group, patients with thin-body habitus, BSA < 1 m2, had no skin tenting or device erosion. Of note, there were 3 patients in the D cohort who were noted to have skin tenting at both implantation and follow-up, but skin tenting in isolation was not considered a reason for extraction. There was no skin tenting documented at follow-up visits for our VP cohort. While this data is encouraging, the low event rate and total enrollment limit definitive statements regarding an odds ratio for skin tenting in D and VP cohorts, respectively.
There were a total of six patients under 5 years old in our study (3VP, 3D). There was no difference in R-wave amplitude or signal degradation in comparison of these techniques for these younger patients. There was one complication within the D technique cohort. This patient had skin tenting at the time of procedure and developed peri-procedural infection, despite receiving prophylactic cephalexin and the same sterile implantation technique. There were no signs of extrusion in this case. The device was removed and re-implanted via D technique days later. The VP technique cohort had no complications, early explantation, or skin tenting.
Our theory is that a narrow chest width in children under 5 years old can predispose this population to skin tenting with D technique. Teenage populations experience breast development and increase in chest wall musculature during puberty that may theoretically lead to lateral device migration. In theory, VP implantation could help avoid both skin tenting and device migration in these populations. However, further investigation is necessary to make definitive statements about D vs VP techniques in these subgroups.
Conclusion
Vertical–parasternal (VP) cardiac monitor implantation is a safe and effective novel surgical technique. Long-term follow-up of VP-implanted devices demonstrate equivalent R-wave detection and no significant signal deterioration across all age groups and body surface areas, when compared to the currently recommended implantation technique. Further investigation using larger cohort studies will be necessary to elucidate difference in complication rate between D and VP cohorts, given low complication incidence. VP implantation technique should be considered a potential alternative to manufacturer-recommended D implantation technique.
References
MacCormick JM, Crawford JR, Chung SK et al (2011) Symptoms and signs associated with syncope in young people with primary cardiac arrhythmias. Heart Lung Circ 20(9):593–598. https://doi.org/10.1016/j.hlc.2011.04.036
Sulke N, Sugihara C, Hong P, Patel N, Freemantle N (2016) The benefit of a remotely monitored implantable loop recorder as a first line investigation in unexplained syncope: the EaSyAS II trial. Europace 6:912–918. https://doi.org/10.1093/europace/euv228
Dwivedi A, Joza J, Malkani K, Mendelson TB, Priori SG, Chinitz LA, Fowler SJ, Cerrone M (2018) Implantable loop recorder in inherited arrhythmia diseases. JACC: Clin Electrophysiol 4(10):1372–1374. https://doi.org/10.1016/j.jacep.2018.07.008
Krahn AD et al (1995) The etiology of syncope in patients with negative tilt table and electrophysiological testing. Circulation 92(7):1819–1824. https://doi.org/10.1161/01.cir.92.7.1819
Bloemers BL, Sreeram N (2002) Implantable loop recorders in pediatric practice. J Electrocardiol 35(Suppl):131–135. https://doi.org/10.1054/jelc.2002.37170
Vilcant V, Kousa O, Hai O. Implantable Loop Recorder. (2021). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan Available from: https://www.ncbi.nlm.nih.gov/books/NBK470398/
Yoon JG, Fares M, Hoyt W Jr, Snyder CS (2021) Diagnostic accuracy and safety of confirm Rx™ insertable cardiac monitor in pediatric patients. Pediatr Cardiol 1:142–147. https://doi.org/10.1007/s00246-020-02463-3
Chaouki AS, Czosek RJ, Spar DS (2016) Missing LINQ: extrusion of a new-generation implantable loop recorder in a child. Cardiol Young 7:1445–1447. https://doi.org/10.1017/S1047951116000913
Zakhar J, Blount TJ, Gehi AK, Ferns SJ (2020) Un-LINQed: Spontaneous extrusion of newer generation implantable loop recorders. Indian Pacing Electrophysiol J 20(5):189–192. https://doi.org/10.1016/j.ipej.2020.04.005
Bezzerides VJ, Walsh A, Martuscello M, Escudero CA, Gauvreau K, Lam G, Abrams DJ, Triedman JK, Alexander ME, Bevilacqua L, Mah DY (2019) The real-world utility of the LINQ implantable loop recorder in pediatric and adult congenital heart patients. JACC Clin Electrophysiol 2:245–251. https://doi.org/10.1016/j.jacep.2018.09.016
Silvetti MS, Tamburri I, Porco L, Saputo FA, Di Mambro C, Righi D, Cazzoli I, Cicenia M, Campisi M, Ravà L, Pizzicaroli C, Drago F (2022) A decade of insertable cardiac monitors with remote monitoring in pediatric patients. Rev Cardiovasc Med 23(1):27. https://doi.org/10.31083/j.rcm2301027
Author information
Authors and Affiliations
Contributions
Peter Woolman performed all data processing, retrospective chart review, and wrote the main manuscript text. Justin Yoon performed background research with the pediatric electrophysiology department that helped inspire this study. Christopher Snyder conceived this project and served as both mentor and project editor from conception to completion of the study.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Woolman, P., Yoon, J. & Snyder, C. Novel Technique for Cardiac Monitor Implantation in Pediatrics. Pediatr Cardiol 44, 141–145 (2023). https://doi.org/10.1007/s00246-022-02974-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-022-02974-1