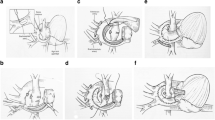
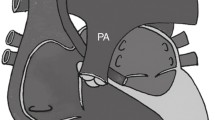
Abstract
Mortality in infants with hypoplastic left heart syndrome (HLHS) is strongly correlated with right ventricle (RV) dysfunction. Cell therapy has demonstrated potential improvements of RV dysfunction in animal models related to HLHS, and neonatal human derived c-kit+ cardiac-derived progenitor cells (CPCs) show superior efficacy when compared to adult human cardiac-derived CPCs (aCPCs). Neonatal CPCs (nCPCs) have yet to be investigated in humans. The CHILD trial (Autologous Cardiac Stem Cell Injection in Patients with Hypoplastic Left Heart Syndrome) is a Phase I/II trial aimed at investigating intramyocardial administration of autologous nCPCs in HLHS infants by assessing the feasibility, safety, and potential efficacy of CPC therapy. Using an open-label, multicenter design, CHILD investigates nCPC safety and feasibility in the first enrollment group (Group A/Phase I). In the second enrollment group, CHILD uses a randomized, double-blinded, multicenter design (Group B/Phase II), to assess nCPC efficacy based on RV functional and structural characteristics. The study plans to enroll 32 patients across 4 institutions: Group A will enroll 10 patients, and Group B will enroll 22 patients. CHILD will provide important insights into the therapeutic potential of nCPCs in patients with HLHS.
Clinical Trial Registration https://clinicaltrials.gov/ct2/home NCT03406884, First posted January 23, 2018.

Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Yabrodi M, Mastropietro CW (2017) Hypoplastic left heart syndrome: from comfort care to long-term survival. Pediatr Res 81:142–149. https://doi.org/10.1038/pr.2016.194
Ambastha C, Bittle GJ, Morales D, Parchment N, Saha P, Mishra R, Sharma S, Vasilenko A, Gunasekaran M, Al-Suqi MT, Li D, Yang P, Kaushal S (2018) Regenerative medicine therapy for single ventricle congenital heart disease. Transl Pediatr 7:176–187. https://doi.org/10.21037/tp.2018.04.01
Bittle GJ, Morales D, Deatrick KB, Parchment N, Saha P, Mishra R, Sharma S, Pietris N, Vasilenko A, Bor C, Ambastha C, Gunasekaran M, Li D, Kaushal S (2018) Stem cell therapy for hypoplastic left heart syndrome: mechanism, clinical application, and future directions. Circ Res 123:288–300. https://doi.org/10.1161/CIRCRESAHA.117.311206
Saraf A, Book WM, Nelson TJ, Xu C (2019) Hypoplastic left heart syndrome: from bedside to bench and back. J Mol Cell Cardiol 135:109–118. https://doi.org/10.1016/j.yjmcc.2019.08.005
Williams DL, Gelijns AC, Moskowitz AJ, Weinberg AD, Ng JH, Crawford E, Hayes CJ, Quaegebeur JM (2000) Hypoplastic left heart syndrome: valuing the survival. J Thorac Cardiovasc Surg 119:720–731. https://doi.org/10.1016/S0022-5223(00)70007-9
Altmann K, Printz BF, Solowiejczky DE, Gersony WM, Quaegebeur J, Apfel HD (2000) Two-dimensional echocardiographic assessment of right ventricular function as a predictor of outcome in hypoplastic left heart syndrome. Am J Cardiol 86:964–968. https://doi.org/10.1016/s0002-9149(00)01131-0
Ohye RG, Schranz D, D’Udekem Y (2016) Current therapy for hypoplastic left heart syndrome and related single ventricle lesions. Circulation 134:1265–1279. https://doi.org/10.1161/CIRCULATIONAHA.116.022816
Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW, Investigators PHN (2010) Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med 362:1980–1992. https://doi.org/10.1056/NEJMoa0912461
Feinstein JA, Benson DW, Dubin AM, Cohen MS, Maxey DM, Mahle WT, Pahl E, Villafaňe J, Bhatt AB, Peng LF, Johnson BA, Marsden AL, Daniels CJ, Rudd NA, Caldarone CA, Mussatto KA, Morales DL, Ivy DD, Gaynor JW, Tweddell JS, Deal BJ, Furck AK, Rosenthal GL, Ohye RG, Ghanayem NS, Cheatham JP, Tworetzky W, Martin GR (2012) Hypoplastic left heart syndrome: current considerations and expectations. J Am Coll Cardiol 59(1 Suppl):S1-42. https://doi.org/10.1016/j.jacc.2011.09.022
Brown MA, Rajamarthandan S, Francis B, O’Leary-Kelly MK, Sinha P (2020) Update on stem cell technologies in congenital heart disease. J Card Surg 35:174–179. https://doi.org/10.1111/jocs.14312
Menasché P (2018) Cell therapy trials for heart regeneration - lessons learned and future directions. Nat Rev Cardiol 15:659–671. https://doi.org/10.1038/s41569-018-0013-0
Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J (2013) Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation 128:122–131. https://doi.org/10.1161/CIRCULATIONAHA.112.001075
Sharma S, Mishra R, Bigham GE, Wehman B, Khan MM, Xu H, Saha P, Goo YA, Datla SR, Chen L, Tulapurkar ME, Taylor BS, Yang P, Karathanasis S, Goodlett DR, Kaushal S (2017) A deep proteome analysis identifies the complete secretome as the functional unit of human cardiac progenitor cells. Circ Res 120:816–834. https://doi.org/10.1161/CIRCRESAHA.116.309782
Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, Goh SK, Walker BL, Almeida-Porada G, Wang D, Backer CL, Dudley SC Jr, Wold LE, Kaushal S (2011) Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation 123:364–373. https://doi.org/10.1161/CIRCULATIONAHA.110.971622
Hatzistergos KE, Takeuchi LM, Saur D, Seidler B, Dymecki SM, Mai JJ, White IA, Balkan W, Kanashiro-Takeuchi RM, Schally AV, Hare JM (2015) cKit+ cardiac progenitors of neural crest origin. Proc Natl Acad Sci USA 112:13051–13056
Hatzistergos KE, Durante MA, Valasaki K, Wanschel AC, Harbour JW, Hare JM (2020) A novel cardiomyogenic role for Isl1+ neural crest cells in the inflow tract. Sci Adv 6:eaba9950
van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marbán E, Molkentin JD (2014) c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509:337–341
Simpson DL, Mishra R, Sharma S, Goh SK, Deshmukh S, Kaushal S (2012) A strong regenerative ability of cardiac stem cells derived from neonatal hearts. Circulation 126(11 Suppl 1):S46-53. https://doi.org/10.1161/CIRCULATIONAHA.111.084699
Agarwal U, Smith AW, French KM, Boopathy AV, George A, Trac D, Brown ME, Shen M, Jiang R, Fernandez JD, Kogon BE, Kanter KR, Alsoufi B, Wagner MB, Platt MO, Davis ME (2016) Age-dependent effect of pediatric cardiac progenitor cells after juvenile heart failure. Stem Cells Transl Med 5:883–892. https://doi.org/10.5966/sctm.2015-0241
Wehman B, Pietris N, Bigham G, Siddiqui O, Mishra R, Li T, Aiello E, Jack G, Wang W, Murthi S, Sharma S, Kaushal S (2017) Cardiac progenitor cells enhance neonatal right ventricular function after pulmonary artery banding. Ann Thorac Surg 104:2045–2053. https://doi.org/10.1016/j.athoracsur.2017.04.058
Wehman B, Sharma S, Pietris N, Mishra R, Siddiqui OT, Bigham G, Li T, Aiello E, Murthi S, Pittenger M, Griffith B, Kaushal S (2016) Mesenchymal stem cells preserve neonatal right ventricular function in a porcine model of pressure overload. Am J Physiol Heart Circ Physiol 310:H1816-1826. https://doi.org/10.1152/ajpheart.00955.2015
Davies B, Elwood NJ, Li S, Cullinane F, Edwards GA, Newgreen DF, Brizard CP (2010) Human cord blood stem cells enhance neonatal right ventricular function in an ovine model of right ventricular training. Ann Thorac Surg 89(585–593):593.e1–4. https://doi.org/10.1016/j.athoracsur.2009.10.035
Bolli R, Hare JM, March KL, Pepine CJ, Willerson JT, Perin EC, Yang PC, Henry TD, Traverse JH, Mitrani RD, Khan A, Hernandez-Schulman I, Taylor DA, DiFede DL, Lima JA, Chuch A, Loughran J, Vojvodic RW, Sayre SL, Bettencourt J, Cohen M, Moyé L, Ebert RF, Simari RD, Cardiovascular Cell Therapy Research Network (CCTRN) (2018) Rationale and design of the CONCERT-HF Trial (Combination of Mesenchymal and c-kit+ cardiac stem cells as regenerative therapy for heart failure). Circ Res 122:1703–1715. https://doi.org/10.1161/CIRCRESAHA.118.312978
Kaushal S, Wehman B, Pietris N, Naughton C, Bentzen SM, Bigham G, Mishra R, Sharma S, Vricella L, Everett AD, Deatrick KB, Huang S, Mehta H, Ravekes WA, Hibino N, Difede DL, Khan A, Hare JM (2017) Study design and rationale for ELPIS: a phase I/IIb randomized pilot study of allogeneic human mesenchymal stem cell injection in patients with hypoplastic left heart syndrome. Am Heart J 192:48–56. https://doi.org/10.1016/j.ahj.2017.06.009
Ishigami S, Ohtsuki S, Eitoku T, Ousaka D, Kondo M, Kurita Y, Hirai K, Fukushima Y, Baba K, Goto T, Horio N, Kobayashi J, Kuroko Y, Kotani Y, Arai S, Iwasaki T, Sato S, Kasahara S, Sano S, Oh H (2017) Intracoronary cardiac progenitor cells in single ventricle physiology: the PERSEUS (Cardiac progenitor cell infusion to treat univentricular heart disease) randomized phase 2 trial. Circ Res 120:1162–1173. https://doi.org/10.1161/CIRCRESAHA.116.310253
Ishigami S, Ohtsuki S, Tarui S, Ousaka D, Eitoku T, Kondo M, Okuyama M, Kobayashi J, Baba K, Arai S, Kawabata T, Yoshizumi K, Tateishi A, Kuroko Y, Iwasaki T, Sato S, Kasahara S, Sano S, Oh H (2015) Intracoronary autologous cardiac progenitor cell transfer in patients with hypoplastic left heart syndrome: the TICAP prospective phase 1 controlled trial. Circ Res 116:653–664. https://doi.org/10.1161/CIRCRESAHA.116.304671
Cnota JF, Allen KR, Colan S, Covitz W, Graham EM, Hehir DA, Levine JC, Margossian R, McCrindle BW, Minich LL, Natarajan S, Richmond ME, Hsu DT, Investigators PHN (2013) Superior cavopulmonary anastomosis timing and outcomes in infants with single ventricle. J Thorac Cardiovasc Surg 145:1288–1296. https://doi.org/10.1016/j.jtcvs.2012.07.069
Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E (2007) Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115:896–908. https://doi.org/10.1161/CIRCULATIONAHA.106.655209
Burkhart HM, Qureshi MY, Rossano JW, Cantero Peral S, O’Leary PW, Hathcock M, Kremers W, Nelson TJ, Wanek HLHS Consortium Clinical Pipeline (2019) Autologous stem cell therapy for hypoplastic left heart syndrome: safety and feasibility of intraoperative intramyocardial injections. J Thorac Cardiovasc Surg 158:1614–1623. https://doi.org/10.1016/j.jtcvs.2019.06.001
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J (2009) Evidence for cardiomyocyte renewal in humans. Science 324:98–102. https://doi.org/10.1126/science.1164680
Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S, Kühn B (2013) Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A 110:1446–1451. https://doi.org/10.1073/pnas.1214608110
Bolli R, Mitrani RD, Hare JM, Pepine CJ, Perin EC, Willerson JT, Traverse JH, Henry TD, Yang PC, Murphy MP, March KL, Schulman IH, Ikram S, Lee DP, O’Brien C, Lima JA, Ostovaneh MR, Ambale-Venkatesh B, Lewis G, Khan A, Bacallao K, Valasaki K, Longsomboon B, Gee AP, Richman S, Taylor DA, Lai D, Sayre SL, Bettencourt J, Vojvodic RW, Cohen ML, Simpson L, Aguilar D, Loghin C, Moyé L, Ebert RF, Davis BR, Simari RD, Cardiovascular Cell Therapy Research Network (CCTRN) (2021) A phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur J Heart Fail 23(4):661–674. https://doi.org/10.1002/ejhf.2178
Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A (2012) Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 308:2369–2379. https://doi.org/10.1001/jama.2012.25321
Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL (2005) Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation 112(9 Suppl):I150-156. https://doi.org/10.1161/CIRCULATIONAHA.104.526749
Funding
The CHILD trial is funded through Marcus Foundation, Inc. Sunjay Kaushal is funded by grants from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (R01HL118491, R01 HL139060-01A1, R42HL131226-01); Moseley Foundation; and Maryland Stem Cell Research. Joshua M. Hare is funded by grants from the NIH/National Heart, Lung, and Blood Institute (R01 HL107110, 1R01HL134558-01, 4R01HL084275-10, 5R01HL116899-04, and HHSN268201600012I); NIH/National Cancer Institute (5R01CA136387-07); Soffer Foundation; and Sarr Foundation. Ming-Sing Si is funded by NIH grant K08HL146351.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Joshua M. Hare reports having a patent for cardiac cell-based therapy and holds equity in Vestion Inc. and maintains a professional relationship with Vestion Inc. as a consultant and member of the Board of Directors and Scientific Advisory Board. Vestion Inc. did not play a role in the design, conduct, or funding of the study. Dr. Joshua Hare is the Chief Scientific Officer, a compensated consultant and board member for Longeveron Inc. and holds equity in Longeveron. Dr. Hare is also the co-inventor of intellectual property licensed to Longeveron. Longeveron did not play a role in the design, conduct, or funding of the study. The University of Miami is an equity owner in Longeveron Inc., which has licensed intellectual property from the University of Miami. Sunjay Kaushal is a founder of Neoprogen, Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaushal, S., Hare, J.M., Shah, A.M. et al. Autologous Cardiac Stem Cell Injection in Patients with Hypoplastic Left Heart Syndrome (CHILD Study). Pediatr Cardiol 43, 1481–1493 (2022). https://doi.org/10.1007/s00246-022-02872-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-022-02872-6