Abstract
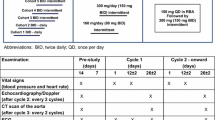
MEK inhibitors (MEKi) have shown efficacy in pediatric low-grade glioma as well as plexiform neurofibroma. MEKi have been associated with acute cardiac dysfunction in adults. Cardiac consequences in children are unknown. We performed a single center retrospective cohort study evaluating cardiac function by echocardiography (echo) in children and young adults < 21 years receiving MEKi between October 2013 and May 2018. Blinded assessment of left ventricular function by fractional shortening (FS) and ejection fraction (EF) was performed on all available echocardiograms performed before, during, and following therapy, as well as after re-initiation of therapy. Twenty-six patients underwent MEKi therapy with echo follow-up during the study period. Twenty-four of these had complete echo data. Median follow-up was 12 months. Borderline EF (EF 53–57.9%) occurred in 12 (50%) patients; and 3 (12.5%) progressed to abnormal EF (EF < 53%). Cardiac dysfunction, when it occurred, was mild (lowest documented EF was 45%, and lowest FS was 24.4%). EF abnormalities typically fluctuated during therapy, resolved off therapy, and recurred with MEKi re-initiation. No clinical or demographic differences were detected between those who maintained normal cardiac function and those who developed borderline or overt cardiac dysfunction. Symptomatic heart failure did not occur. In this cohort of children and young adults, MEKi use was associated with a high (50%) incidence of borderline or mildly decreased left ventricular function. There was no evidence of permanent cardiac dysfunction. Further evaluation in larger prospective trials is needed.


Similar content being viewed by others
Data Availability
Raw data supporting the findings of this study are available from the corresponding authors upon request.
References
Garcia MA, Solomon DA, Haas-Kogan DA (2016) Exploiting molecular biology for diagnosis and targeted management of pediatric low-grade gliomas. Future Oncol 12:1493–1506. https://doi.org/10.2217/fon-2016-0039
Walker JA, Upadhyaya M (2018) Emerging therapeutic targets for neurofibromatosis type 1. Expert Opin Ther Targets 22:419–437. https://doi.org/10.1080/14728222.2018.1465931
Dombi E, Baldwin A, Marcus LJ et al (2016) Activity of selumetinib in neurofibromatosis type 1–related plexiform neurofibromas. N Engl J Med 375:2550–2560. https://doi.org/10.1056/NEJMoa1605943
Banerjee A, Jakacki RI, Onar-Thomas A et al (2017) A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro-Oncol 19:1135–1144. https://doi.org/10.1093/neuonc/now282
Fangusaro J, Onar-Thomas A, Young Poussaint T et al (2019) Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol 20:1011–1022. https://doi.org/10.1016/S1470-2045(19)30277-3
Gross AM, Wolters PL, Dombi E et al (2020) Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med 382:1430–1442. https://doi.org/10.1056/NEJMoa1912735
Office of the Commissioner (2020) FDA approves first therapy for children with debilitating and disfiguring rare disease. In: FDA. Available from https://www.fda.gov/news-events/press-announcements/fda-approves-first-therapy-children-debilitating-and-disfiguring-rare-disease. Accessed May 15, 2020
Abdel-Rahman O, ElHalawani H, Ahmed H (2015) Risk of selected cardiovascular toxicities in patients with cancer treated with MEK inhibitors: a comparative systematic review and meta-analysis. J Glob Oncol 1:73–82. https://doi.org/10.1200/JGO.2015.000802
Banks M, Crowell K, Proctor A, Jensen BC (2017) Cardiovascular effects of the MEK inhibitor, trametinib: a case report, literature review, and consideration of mechanism. Cardiovasc Toxicol 17:487–493. https://doi.org/10.1007/s12012-017-9425-z
Mincu RI, Mahabadi AA, Michel L et al (2019) Cardiovascular adverse events associated with BRAF and MEK inhibitors: a systematic review and meta-analysis. JAMA Netw Open 2:e198890–e198890. https://doi.org/10.1001/jamanetworkopen.2019.8890
Buccheri S, Costanzo L, Tamburino C, Monte I (2015) Reference values for real time three-dimensional echocardiography-derived left ventricular volumes and ejection fraction: review and meta-analysis of currently available studies. Echocardiogr Mt Kisco N 32:1841–1850. https://doi.org/10.1111/echo.12972
Flaherty KT, Infante JR, Daud A et al (2012) Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367:1694–1703. https://doi.org/10.1056/NEJMoa1210093
Flaherty KT, Robert C, Hersey P et al (2012) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367:107–114. https://doi.org/10.1056/NEJMoa1203421
Patnaik A, Tolcher A, Papadopoulos KP et al (2016) Phase 1 study to evaluate the effect of the MEK inhibitor trametinib on cardiac repolarization in patients with solid tumours. Cancer Chemother Pharmacol 78:491–500. https://doi.org/10.1007/s00280-016-3090-y
Chang F, Steelman LS, Shelton JG et al (2003) Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (review). Int J Oncol 22:469–480. https://doi.org/10.3892/ijo.22.3.469
Khalilimeybodi A, Daneshmehr A, Sharif-Kashani B (2018) Investigating β-adrenergic-induced cardiac hypertrophy through computational approach: classical and non-classical pathways. J Physiol Sci 68:503–520. https://doi.org/10.1007/s12576-017-0557-5
Rezatabar S, Karimian A, Rameshknia V et al (2019) RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. J Cell Physiol 234:14951–14965. https://doi.org/10.1002/jcp.28334
Fischer P, Hilfiker-Kleiner D (2007) Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic Res Cardiol 102:393–411. https://doi.org/10.1007/s00395-007-0674-z
Aikawa R, Komuro I, Yamazaki T et al (1997) Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest 100:1813–1821
Vajravelu BN, Hong KU, Al-Maqtari T et al (2015) C-kit promotes growth and migration of human cardiac progenitor cells via the PI3K-AKT and MEK-ERK pathways. PLoS ONE 10:e0140798. https://doi.org/10.1371/journal.pone.0140798
Xia P, Liu Y, Cheng Z (2016) Signaling pathways in cardiac myocyte apoptosis. BioMed Res Int 2016:9583268. https://doi.org/10.1155/2016/9583268
Yue T-L, Wang C, Gu J-L et al (2000) Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation–induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res 86:692–699. https://doi.org/10.1161/01.RES.86.6.692
Zhang Y, Zhang X-F, Gao L et al (2014) Growth/differentiation factor 1 alleviates pressure overload-induced cardiac hypertrophy and dysfunction. Biochim Biophys Acta BBA—Mol Basis Dis 1842:232–244. https://doi.org/10.1016/j.bbadis.2013.11.018
Bisserier M, Berthouze-Duquesnes M, Breckler M et al (2015) Carabin protects against cardiac hypertrophy by blocking calcineurin, Ras, and Ca2+/calmodulin-dependent protein kinase II signaling clinical perspective. Circulation 131:390–400. https://doi.org/10.1161/CIRCULATIONAHA.114.010686
Cipolletta E, Rusciano MR, Maione AS et al (2015) Targeting the CaMKII/ERK Interaction in the Heart Prevents Cardiac Hypertrophy. PLoS ONE 10(6):e0130477. https://doi.org/10.1371/journal.pone.0130477
Cichowski K, Jänne PA (2010) Drug discovery: inhibitors that activate. Nature 464:358–359. https://doi.org/10.1038/464358a
Zhang C, Spevak W, Zhang Y et al (2015) RAF inhibitors that evade paradoxical MAPK pathway activation. Nature 526:583–586. https://doi.org/10.1038/nature14982
Acknowledgements
The authors would like to thank Ashley S. Margol, Tom Belle Davidson, Anat Erdreich-Epstein, Kasey Rangan, Kaaren Waters, and Kimberly Bira for their intellectual contributions and assistance with data collection. The authors would also like to thank Cecilia Patino-Sutton, Todd Alonzo, and Yueh-Yun Chi for their editorial review of the manuscript drafts. MJF was a participant in the University of Southern California/CHLA Summer Oncology Research Fellowship (SORF) Program.
Author information
Authors and Affiliations
Contributions
Study conception: All authors. Data collection: MJF, NJR. Echocardiogram central review: JAS, JDM. Data analysis and interpretation: All authors. Initial manuscript draft: MJF, NJR, JAS. Revised and approved final manuscript: All authors.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no financial or non-financial conflict of interest.
Ethical Approval
The study was approved by the Children’s Hospital Los Angeles Institutional Review Board (IRB), with waiver of consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Preliminary data were presented under the title “Cardiac Effects of MEK Inhibitors in Children” at the American Heart Association Scientific Sessions, Nov 2019, Philadelphia; meeting abstract published in Circulation. 2019;140:A12608.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Robison, N.J., Su, J.A., Fang, M.J. et al. Cardiac Function in Children and Young Adults Treated with MEK Inhibitors: A Retrospective Cohort Study. Pediatr Cardiol 43, 1223–1228 (2022). https://doi.org/10.1007/s00246-022-02842-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-022-02842-y