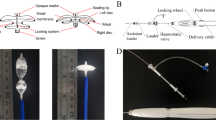
Abstract
To date, there has been limited investigation of bioabsorbable atrial septal defect (ASD) or patent foramen ovale (PFO) closure devices using clinically relevant large animal models. The purpose of this study is to explore the function and safety of a bioabsorbable ASD occluder (BAO) system for PFO and/or secundum ASD transcatheter closure. Using a sheep model, the intra-atrial septum was evaluated by intracardiac echo (ICE). If a PFO was not present, atrial communication was created via transseptal puncture. Device implantation across the intra-atrial communication was performed with fluoroscopic and ICE guidance. Our 1st generation device consisted of a main structure of thin Poly(l-lactide-co-epsilon-caprolactone) (PLCL) fibers, and an internal Poly glycolic acid (PGA) fabric. Four procedures validated procedure feasibility. Subsequently, device design was modified for improved transcatheter delivery. The 2nd generation device has a two-layered structure and was implanted in six sheep. Results showed procedural success in 9/10 (90%) animals. With deployment, the 1st generation device did not reform into its original disk shape and did not conform nicely along the atrial septum. The 2nd generation device was implanted in six animals, 3 out of 6 survived out to 1 year. At 1 year post implantation, ICE confirmed no residual shunting. By necropsy, biomaterials had partially degraded, and histology of explanted samples revealed significant device endothelialization and biomaterial replacement with a collagen layer. Our results demonstrate that our modified 2nd generation BAO can be deployed via minimally invasive percutaneous transcatheter techniques. The BAO partially degrades over 1 year and is replaced by host native tissues. Future studies are needed prior to clinical trials.







Similar content being viewed by others
References
Geva T, Martins JD, Wald RM (2014) Atrial septal defects. Lancet 383:1921–1932
Mai CT, Isenburg JL, Canfield MA et al (2019) National population-based estimates for major birth defects, 2010–2014. Birth Defects Res 111:1420–1435
Homma S, Sacco RL (2005) Patent forman ovale and stroke. Circulation 112:1063–1072
Moore J, Hegde S, El-Said H et al (2013) Transcatheter device closure of atrial septal defects: a safety review. JACC Cardiovasc Int 6:433–442
Kutty S, Hazeem AA, Brown K, Danford CJ, Worley SE, Delaney JW, Danford DA, Latson LA (2012) Long-term outcomes after transcatheteror surgical treatment of hemodynamically significant isolated secundum atrial septal defect. Am J Cardiol 109:1348–1352
Kotowycz MA, Therrien J, Ionescu-Ittu R, Owens CG, Pilote L, Martucci G, Tchervenkov C, Marelli AJ (2013) Long-term outcomes after surgical versus transcatheter closure of atrial septal defects in adults. JACC Cardiovasc Int 6:497–503
Attie F, Rosas M, Granados N, Zabal C, Buendía A, Calderón J (2001) Surgical treatment for secundum atrial septal defects in patients > 40 years old: a randomized clinical trial. J Am Coll Cardiol 38:2035–2042
Vasquez AF, Lasala JM (2013) Atrial septal defect closure. Cardiol Clin 31:385–400
Huang Y, Kong JF, Venkatraman SS (2014) Biomaterials and design in occlusion devices for cardiac defects: a review. Acta Biomater 10:1088–1101
Duong-Hong D, Tang YD, Wu W et al (2010) Fully biodegradable septal defect occluder-a double umbrella designa. Catheter Cardiol Int 76:711–718
Liu SJ, Peng KM, Hsiao CY et al (2011) Novel biodegradable polycaprolactone occlusion device combining nanofibrous PLGA/collagen membrane for closure of atrial septal defect (ASD). Ann Biomed Eng 39:2759–2766
Lu W, Ouyang W, Wang S et al (2018) A novel totally biodegradable device for effective atrial septal defect closure: a 2-year study in sheep. J Interv Cardiol 3:841–848
Saver JL, Carrol JD, Thaler DE et al (2017) Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med 377(11):1022–1032
Happel CM, Laser KT, Sigler M et al (2015) Singlecenter experience: implantation failures, early, and late complications after implantation of a partially biodegradable ASD/PFO-device (Bio Star Ò). Catheter Cardiol Int 85:990–997
Sigler M, Söderberg B, Schmitt B et al (2018) Carag-bioresorbable septal occluder (CBSO): histopathology of experimental implants. Euro Interv 13:1655–1661
Shi D, Kang Y, Zhang G, Gao C, Lu W, Zou H, Jiang H (2019) Biodegradable atrial septal defect occluders: a current review. Acta Biomater 96:68–80
Cruz SO, Flecknell P, Richardson C (2014) Animal research in pediatric cardiology and cardiac surgery. Pediatr Congen Cardiol Cardiac Surg Intensive Care. https://doi.org/10.1007/978-1-4471-4619-3
Patric L, Simon NT, Yvonnick B (2014) Animal models for studies of arterial stiffness. Blood pressure and arterial wall mechanics in cardiovascular disease. Springer, Lodon, pp 63–74
Agarwal R, Blum K, Musgrave A et al (2019) Degradation and in vivo evaluation of polycaprolactone, poly(ε-caprolactone-co-l-lactide), and poly-l-lactic acid as scaffold sealant polymers for murine tissue-engineered vascular grafts. Regen Med 14(7):627–637
Lee SH, Lee JH, Choo YS (2014) Analysis of degradation rate for dimensionless surface area of well-interconnected PCL scaffold via in-vitro accelerated degradation experiment. Tissue Eng Regen Med 11(6):446–452
Rittle L (2017) Method for picrosirius red-polarizattion detections of collagen fibers in tissue sections. Methods Mol Biol 1627:395–407
Viju S, Thilagavathi G (2010) Preparation and properties of PLLA/PLCL fibers for potential use as a monofilament suture. J Textile Inst 101(9):835–841
Ravi S, Chaikof EL (2015) Biomaterials for vascular tissue engineering. Regen Med 5(1):107
Pavcnik D, Tekulve K, Uchida B et al (2012) Double BioDisk: a new bioprosthetic device for transcatheter closure of atrial septal defects-a feasibility study in adult sheep. Radiol Oncol 46:89–96
Xie ZF, Wang SS, Zhang ZW et al (2016) A novel-design poly-l-lactic acid biodegradable device for closure of atrial septal defect: long-term results in swine. Cardiology 135(3):179–187
Naik N (2015) How to perform transeptal puncture. Indian Heart J 67(1):70–76
Acknowledgements
This research is supported by GUNZE Co ltd and The Intramural Heart Center Grant of Nationwide Children’s Specifically, GUNZE Co., Ltd (Saki Okumura M.S, et al.) provided material support through the creation of the Bioabsorbable ASD device. Jason Swinning RT(R) (CI), RCIS as a radiologist was a tremendous contributor to the establishment of this animal model. YM was supported by Department of Defense (DoD) and Funding award from Uehara Memorial Foundation (Tokyo Japan) in 2019. HK was supported by Health Labour Sciences Research Grant and AMED under Grant Number JP15hk0102006. The specific roles of these authors are articulated in the 'author contributions' section.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have read the journal's policy and the authors of the manuscript have the following competing interests: TS has received grant support from Gunze Ltd. No authors received salary support from Gunze Ltd. This does not alter our adherence to Pediatric Cardiology policies on sharing data and materials. There are no patents, products in development, or marketed products to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PPTX 2274 KB)
Supplement 1: Intra Cardiac Echo imaging. ICE images above reveal the created PFO (a) and no residual shunting following placement of the device (b,c) (atrial disks outlined in red). Supplement 2: In vitro hydrolysis study of 1st and 2nd generation ASD devices. The ASD devices were immersed in PBS solution and the mass of the excised samples were compared to Day 0. (a) 1st generation device, (b) 2nd generation device. Supplement 3: Surgical ASD model under cardio-pulmonary bypass was performed on 4 lambs. After making the ASD, our biodegradable devices were implanted as ASD occludesr. There were no deaths in the acute and chronic phases after surgery. Supplement 4 (a) Image of the modified 1st generation ASD device at 1 year timepoint. (b) Fluoro image of modified 1st generation device at 1 year timepoint. (c )Histological evaluation of modified 1st generation ASD device. The red scale bar is 2000μm. 5x magnification. (d) Immunohistochemistry against SMA, vWF, CD68. The scale bar is 200 μm. 10x maginification. Supplement 5: Histology of lungs and brains of sheep implanted with ASD device (1 year timepoint).There was no evidence of infarction by histological analysis. The scale bar is 200 μm. 5x magnification.
Rights and permissions
About this article
Cite this article
Matsuzaki, Y., Berman, D.P., Kurobe, H. et al. Pre-clinical Evolution of a Novel Transcatheter Bioabsorbable ASD/PFO Occluder Device. Pediatr Cardiol 43, 986–994 (2022). https://doi.org/10.1007/s00246-021-02809-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-021-02809-5