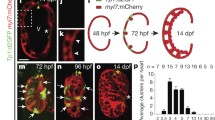
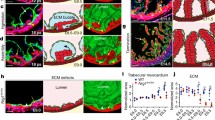
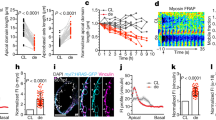
Abstract
Trabecular morphogenesis is a key morphologic event during cardiogenesis and contributes to the formation of a competent ventricular wall. Lack of trabeculation results in embryonic lethality. The trabecular morphogenesis is a multistep process that includes, but is not limited to, trabecular initiation, proliferation/growth, specification, and compaction. Although a number of signaling molecules have been implicated in regulating trabeculation, the cellular processes underlying mammalian trabecular formation are not fully understood. Recent works show that the myocardium displays polarity, and oriented cell division (OCD) and directional migration of the cardiomyocytes in the monolayer myocardium are required for trabecular initiation and formation. Furthermore, perpendicular OCD is an extrinsic asymmetric cell division that contributes to trabecular specification, and is a mechanism that causes the trabecular cardiomyocytes to be distinct from the cardiomyocytes in compact zone. Once the coronary vasculature system starts to function in the embryonic heart, the trabeculae will coalesce with the compact zone to thicken the heart wall, and abnormal compaction will lead to left ventricular non-compaction (LVNC) and heart failure. There are many reviews about compaction and LVNC. In this review, we will focus on the roles of myocardial polarity and OCD in trabecular initiation, formation, and specification.





Similar content being viewed by others
References
Manasek FJ (1968) Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. J Morphol 125:329–365. https://doi.org/10.1002/jmor.1051250306
Van Mierop LH (1979) Embryology of the univentricular heart. Herz 4:78–85
Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH (2000) Developmental patterning of the myocardium. Anat Rec 258:319–337
Icardo JM, Fernandez-Teran A (1987) Morphologic study of ventricular trabeculation in the embryonic chick heart. Acta Anat 130:264–274
Jenni R, Rojas J, Oechslin E (1999) Isolated noncompaction of the myocardium. N Engl J Med 340:966–967. https://doi.org/10.1056/NEJM199903253401215
Gassmann M et al (1995) Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378:390–394. https://doi.org/10.1038/378390a0
Jefferies JL et al (2015) Cardiomyopathy phenotypes and outcomes for children with left ventricular myocardial noncompaction: results from the pediatric cardiomyopathy registry. J Cardiac Fail 21:877–884. https://doi.org/10.1016/j.cardfail.2015.06.381
Towbin JA, Jefferies JL (2017) Cardiomyopathies due to left ventricular noncompaction, mitochondrial and storage diseases, and inborn errors of metabolism. Circ Res 121:838–854. https://doi.org/10.1161/CIRCRESAHA.117.310987
Finsterer J (2010) Left ventricular non-compaction and its cardiac and neurologic implications. Heart Fail Rev 15:589–603. https://doi.org/10.1007/s10741-010-9175-5
Hussein A, Karimianpour A, Collier P, Krasuski RA (2015) Isolated noncompaction of the left ventricle in adults. J Am Coll Cardiol 66:578–585. https://doi.org/10.1016/j.jacc.2015.06.017
Weiford BC, Subbarao VD, Mulhern KM (2004) Noncompaction of the ventricular myocardium. Circulation 109:2965–2971. https://doi.org/10.1161/01.CIR.0000132478.60674.D0
Horvitz HR, Herskowitz I (1992) Mechanisms of asymmetric cell division: two Bs or not two Bs, that is the question. Cell 68:237–255
Jan YN, Jan LY (2000) Polarity in cell division: what frames thy fearful asymmetry?. Cell 100:599–602
Neumuller RA, Knoblich JA (2009) Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev 23:2675–2699. https://doi.org/10.1101/gad.1850809
Wu M, Herman MA (2006) A novel noncanonical Wnt pathway is involved in the regulation of the asymmetric B cell division in C. elegans. Dev Biol 293:316–329. https://doi.org/10.1016/j.ydbio.2005.12.024
Bryant DM, Mostov KE (2008) From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol 9:887–901. https://doi.org/10.1038/nrm2523
Wu M et al (2010) Epicardial spindle orientation controls cell entry into the myocardium. Dev Cell 19:114–125. https://doi.org/10.1016/j.devcel.2010.06.011
Li J et al (2017) CDC42 is required for epicardial and pro-epicardial development by mediating FGF receptor trafficking to the plasma membrane. Development 144:1635–1647. https://doi.org/10.1242/dev.147173
Hirose T et al (2006) PAR3 is essential for cyst-mediated epicardial development by establishing apical cortical domains. Development 133:1389–1398. https://doi.org/10.1242/dev.02294
Rhee DY et al (2009) Connexin 43 regulates epicardial cell polarity and migration in coronary vascular development. Development 136:3185–3193. https://doi.org/10.1242/dev.032334
Sengbusch JK, He W, Pinco KA, Yang JT (2002) Dual functions of α4β1 integrin in epicardial development: initial migration and long-term attachment. J Cell Biol 157:873–882. https://doi.org/10.1083/jcb.200203075
Jimenez-Amilburu V et al (2016) In vivo visualization of cardiomyocyte apicobasal polarity reveals epithelial to mesenchymal-like transition during cardiac trabeculation. Cell Rep 17:2687–2699. https://doi.org/10.1016/j.celrep.2016.11.023
Passer D, van de Vrugt A, Atmanli A, Domian IJ (2016) Atypical protein kinase C-dependent polarized cell division is required for myocardial trabeculation. Cell Rep 14:1662–1672. https://doi.org/10.1016/j.celrep.2016.01.030
Li J et al (2016) Single-cell lineage tracing reveals that oriented cell division contributes to trabecular morphogenesis and regional specification. Cell Rep 15:158–170. https://doi.org/10.1016/j.celrep.2016.03.012
Castanon I, Gonzalez-Gaitan M (2011) Oriented cell division in vertebrate embryogenesis. Curr Opin Cell Biol 23:697–704. https://doi.org/10.1016/j.ceb.2011.09.009
Wei Y, Mikawa T (2000) Formation of the avian primitive streak from spatially restricted blastoderm: evidence for polarized cell division in the elongating streak. Development 127:87–96
Baena-Lopez LA, Baonza A, Garcia-Bellido A (2005) The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol 15:1640–1644. https://doi.org/10.1016/j.cub.2005.07.062
Liu J et al (2010) A dual role for ErbB2 signaling in cardiac trabeculation. Development 137:3867–3875. https://doi.org/10.1242/dev.053736
Han P et al (2016) Coordinating cardiomyocyte interactions to direct ventricular chamber morphogenesis. Nature 534:700–704. https://doi.org/10.1038/nature18310
Staudt DW et al (2014) High-resolution imaging of cardiomyocyte behavior reveals two distinct steps in ventricular trabeculation. Development 141:585–593. https://doi.org/10.1242/dev.098632
Meilhac SM, Esner M, Kerszberg M, Moss JE, Buckingham ME (2004) Oriented clonal cell growth in the developing mouse myocardium underlies cardiac morphogenesis. J Cell Biol 164:97–109. https://doi.org/10.1083/jcb.200309160
Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME (2004) The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell 6:685–698
Meilhac SM et al (2003) A retrospective clonal analysis of the myocardium reveals two phases of clonal growth in the developing mouse heart. Development 130:3877–3889
Mikawa T, Cohen-Gould L, Fischman DA (1992) Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus. III: polyclonal origin of adjacent ventricular myocytes. Dev Dyn 195:133–141. https://doi.org/10.1002/aja.1001950208
Du Q, Stukenberg PT, Macara IG (2001) A mammalian Partner of inscuteable binds NuMA and regulates mitotic spindle organization. Nat Cell Biol 3:1069–1075. https://doi.org/10.1038/ncb1201-1069
Du Q, Macara IG (2004) Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 119:503–516. https://doi.org/10.1016/j.cell.2004.10.028
Mauser JF, Prehoda KE (2012) Inscuteable regulates the Pins-Mud spindle orientation pathway. PLoS ONE 7:e29611. https://doi.org/10.1371/journal.pone.0029611
Laan L et al (2012) Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell 148:502–514. https://doi.org/10.1016/j.cell.2012.01.007
Seldin L, Muroyama A, Lechler T (2016) NuMA-microtubule interactions are critical for spindle orientation and the morphogenesis of diverse epidermal structures. eLife. https://doi.org/10.7554/eLife.12504
Hendricks AG et al (2012) Dynein tethers and stabilizes dynamic microtubule plus ends. Curr Biol 22:632–637. https://doi.org/10.1016/j.cub.2012.02.023
Yap AS, Brieher WM, Gumbiner BM (1997) Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol 13:119–146. https://doi.org/10.1146/annurev.cellbio.13.1.119
Inaba M, Yuan H, Salzmann V, Fuller MT, Yamashita YM (2010) E-cadherin is required for centrosome and spindle orientation in Drosophila male germline stem cells. PLoS ONE 5:e12473. https://doi.org/10.1371/journal.pone.0012473
den Elzen N, Buttery CV, Maddugoda MP, Ren G, Yap AS (2009) Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia. Mol Biol Cell 20:3740–3750. https://doi.org/10.1091/mbc.E09-01-0023
Le Borgne R, Bellaiche Y, Schweisguth F (2002) Drosophila E-cadherin regulates the orientation of asymmetric cell division in the sensory organ lineage. Curr Biol 12:95–104 pii]
Lu B, Roegiers F, Jan LY, Jan YN (2001) Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature 409:522–525. https://doi.org/10.1038/35054077
Yamashita YM, Jones DL, Fuller MT (2003) Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301:1547–1550. https://doi.org/10.1126/science.1087795
Gloerich M, Bianchini JM, Siemers KA, Cohen DJ, Nelson WJ (2017) Cell division orientation is coupled to cell–cell adhesion by the E-cadherin/LGN complex. Nat Commun 8:13996. https://doi.org/10.1038/ncomms13996
Luo Y et al (2001) Rescuing the N-cadherin knockout by cardiac-specific expression of N- or E-cadherin. Development 128:459–469
Cherian AV, Fukuda R, Augustine SM, Maischein HM, Stainier DY (2016) N-cadherin relocalization during cardiac trabeculation. Proc Natl Acad Sci USA 113:7569–7574. https://doi.org/10.1073/pnas.1606385113
Sedmera D et al (2003) Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat Rec 274:773–777. https://doi.org/10.1002/ar.a.10085
Zhang W, Chen H, Qu X, Chang CP, Shou W (2013) Molecular mechanism of ventricular trabeculation/compaction and the pathogenesis of the left ventricular noncompaction cardiomyopathy (LVNC). Am J Med Genet Part C 163:144–156. https://doi.org/10.1002/ajmg.c.31369
Kochilas LK, Li J, Jin F, Buck CA, Epstein JA (1999) p57Kip2 expression is enhanced during mid-cardiac murine development and is restricted to trabecular myocardium. Pediatr Res 45:635–642
Chen H et al (2004) BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development 131:2219–2231. https://doi.org/10.1242/dev.01094
Clay H et al (2016) Sphingosine 1-phosphate receptor-1 in cardiomyocytes is required for normal cardiac development. Dev Biol 418:157–165. https://doi.org/10.1016/j.ydbio.2016.06.024
Miao L et al (2018) Notch signaling regulates Hey2 expression in a spatiotemporal dependent manner during cardiac morphogenesis and trabecular specification. Sci Rep 8:2678. https://doi.org/10.1038/s41598-018-20917-w
Xin M et al (2007) Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc Natl Acad Sci USA 104:7975–7980. https://doi.org/10.1073/pnas.0702447104
Koibuchi N, Chin MT (2007) CHF1/Hey2 plays a pivotal role in left ventricular maturation through suppression of ectopic atrial gene expression. Circ Res 100:850–855. https://doi.org/10.1161/01.RES.0000261693.13269.bf
Kokubo H et al (2004) Targeted disruption of hesr2 results in atrioventricular valve anomalies that lead to heart dysfunction. Circ Res 95:540–547. https://doi.org/10.1161/01.RES.0000141136.85194.f0
Lee KF et al (1995) Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 378:394–398. https://doi.org/10.1038/378394a0
Meyer D, Birchmeier C (1995) Multiple essential functions of neuregulin in development. Nature 378:386–390. https://doi.org/10.1038/378386a0
Grego-Bessa J et al (2007) Notch signaling is essential for ventricular chamber development. Dev Cell 12:415–429. https://doi.org/10.1016/j.devcel.2006.12.011
VanDusen NJ et al (2014) Hand2 is an essential regulator for two Notch-dependent functions within the embryonic endocardium. Cell Rep 9:2071–2083. https://doi.org/10.1016/j.celrep.2014.11.021
Xin M et al (2011) Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal 4:ra70. https://doi.org/10.1126/scisignal.2002278
von Gise A et al (2012) YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci USA 109:2394–2399. https://doi.org/10.1073/pnas.1116136109
Li D et al (2011) Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development 138:303–315. https://doi.org/10.1242/dev.055566
Zhao C et al (2014) Numb family proteins are essential for cardiac morphogenesis and progenitor differentiation. Development 141:281–295. https://doi.org/10.1242/dev.093690
Wu M, Li J (2015) Numb family proteins: novel players in cardiac morphogenesis and cardiac progenitor cell differentiation. Biomol Concepts 6:137–148. https://doi.org/10.1515/bmc-2015-0003
Tian X et al (2017) Identification of a hybrid myocardial zone in the mammalian heart after birth. Nat Commun 8:87. https://doi.org/10.1038/s41467-017-00118-1
Acknowledgements
We thank the Wu laboratory members for scientific discussion, and Dr. John Schwarz for critical reading.
Funding
This study was funded by American Heart Association [13SDG16920099] and by National Heart, Lung, and Blood Institute Grant [R01HL121700] to MW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no competing interests.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Research Involving Human Participants
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Wu, M. Mechanisms of Trabecular Formation and Specification During Cardiogenesis. Pediatr Cardiol 39, 1082–1089 (2018). https://doi.org/10.1007/s00246-018-1868-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-018-1868-x