Abstract
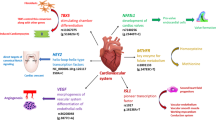
Transforming growth factor beta receptor 2 (TGFBR2) plays a central role in normal heart development, and we investigated whether TGFBR2 polymorphism confers the risk of congenital ventricular septal defect (CVSD). The case–control study included 115 CVSD children and 188 healthy children in a Chinese population. TGFBR2 rs6785358 polymorphism was genotyped with polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP). Enzyme-linked immunoassay (ELISA) was used to detect serum TGFBR2 levels. The genotype and allele frequency of TGFBR2 rs6785358 were significantly higher in the CVSD group than in the controls (all P < 0.05). The G allele carriers were associated with increased CVSD risk compared with the A allele carriers in CVSD group (OR 3.503, 95 % CI 2.670–4.596). Stratified analysis by gender revealed that the TGFBR2 rs6785358 genotype and allele frequency were significantly different between the CVSD case and controls, in both the male subgroup and the female subgroup (all P < 0.001). The G allele carriers were more susceptible to CVSD risk than the A allele carriers in both the male subgroup (OR 9.096, 95 % CI 5.398–15.33) and the female subgroup (OR 3.148, 95 % CI 1.764–5.618). Logistic regression analysis revealed that age, gender and genotype were associated with the risk of CVSD (all P < 0.05). The study findings revealed that TGFBR2 rs6785358 polymorphism contributes to CVSD susceptibility, and the G allele may increase the risk of CVSD.

Similar content being viewed by others
References
Alverson CJ, Strickland MJ, Gilboa SM, Correa A (2011) Maternal smoking and congenital heart defects in the Baltimore-Washington Infant Study. Pediatrics 127(3):e647–e653
Azhar M, Runyan RB, Gard C, Sanford LP, Miller ML, Andringa A et al (2009) Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev Dyn 238(2):431–442
Azhar M, Brown K, Gard C, Chen H, Rajan S, Elliott DA et al (2011) Transforming growth factor Beta2 is required for valve remodeling during heart development. Dev Dyn 240(9):2127–2141
Barnett JV, Desgrosellier JS (2003) Early events in valvulogenesis: a signaling perspective. Birth Defects Res C Embryo Today 69(1):58–72
Bettinelli AL, Mulder TJ, Funke BH, Lafferty KA, Longo SA, Niyazov DM (2013) Familial ebstein anomaly, left ventricular hypertrabeculation, and ventricular septal defect associated with a MYH7 mutation. Am J Med Genet A 161A(12):3187–3190
Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S et al (2004) TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303(5659):848–851
Boyer AS, Runyan RB (2001) TGFbeta Type III and TGFbeta Type II receptors have distinct activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Dyn 221(4):454–459
Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB (1999) TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol 208(2):530–545
Buskohl PR, Sun MJ, Thompson RP, Butcher JT (2012) Serotonin potentiates transforming growth factor-beta3 induced biomechanical remodeling in avian embryonic atrioventricular valves. PLoS One 7(8):e42527
Butcher JT, Markwald RR (2007) Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci 362(1484):1489–1503
Cheifetz S, Andres JL, Massague J (1988) The transforming growth factor-beta receptor type III is a membrane proteoglycan. Domain structure of the receptor. J Biol Chem 263(32):16984–16991
Chiu YN, Norris RA, Mahler G, Recknagel A, Butcher JT (2010) Transforming growth factor beta, bone morphogenetic protein, and vascular endothelial growth factor mediate phenotype maturation and tissue remodeling by embryonic valve progenitor cells: relevance for heart valve tissue engineering. Tissue Eng Part A 16(11):3375–3383
Chua KN, Poon KL, Lim J, Sim WJ, Huang RY, Thiery JP (2011) Target cell movement in tumor and cardiovascular diseases based on the epithelial-mesenchymal transition concept. Adv Drug Deliv Rev 63(8):558–567
Correa A, Marcinkevage J (2013) Prepregnancy obesity and the risk of birth defects: an update. Nutr Rev 71(Suppl 1):S68–S77
de Caestecker M (2004) The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev 15(1):1–11
Dolk H, Loane M, Garne E, European Surveillance of Congenital Anomalies Working G (2011) Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation 123(8):841–849
Fahed AC, Gelb BD, Seidman JG, Seidman CE (2013) Genetics of congenital heart disease: the glass half empty. Circ Res 112(4):707–720
Girdauskas E, Schulz S, Borger MA, Mierzwa M, Kuntze T (2011) Transforming growth factor-beta receptor type II mutation in a patient with bicuspid aortic valve disease and intraoperative aortic dissection. Ann Thorac Surg 91(5):e70–e71
Glas J, Seiderer J, Bues S, Stallhofer J, Fries C, Olszak T et al (2013) IRGM variants and susceptibility to inflammatory bowel disease in the German population. PLoS One 8(1):e54338
Greutmann M, Tobler D (2012) Changing epidemiology and mortality in adult congenital heart disease: looking into the future. Future Cardiol 8(2):171–177
Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO (2011) Autocrine transforming growth factor-beta1 promotes in vivo Th17 cell differentiation. Immunity 34(3):396–408
Heldin CH, Miyazono K, ten Dijke P (1997) TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390(6659):465–471
Hoffman J (2013) The global burden of congenital heart disease. Cardiovasc J Afr 24(4):141–145
Hu Z, Shi Y, Mo X, Xu J, Zhao B, Lin Y et al (2013) A genome-wide association study identifies two risk loci for congenital heart malformations in Han Chinese populations. Nat Genet 45(7):818–821
Jaffe M, Sesti C, Washington IM, Du L, Dronadula N, Chin MT et al (2012) Transforming growth factor-beta signaling in myogenic cells regulates vascular morphogenesis, differentiation, and matrix synthesis. Arterioscler Thromb Vasc Biol 32(1):e1–e11
Jiao K, Langworthy M, Batts L, Brown CB, Moses HL, Baldwin HS (2006) Tgfbeta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development 133(22):4585–4593
Knapczyk-Stwora K, Grzesiak M, Duda M, Koziorowski M, Galas J, Slomczynska M (2014) TGFbeta (transforming growth factor beta) superfamily members and their receptors in the fetal porcine ovaries: effect of prenatal flutamide treatment. Folia Histochem Cytobiol 52(4):317–325
Kruithof BP, Duim SN, Moerkamp AT, Goumans MJ (2012) TGFbeta and BMP signaling in cardiac cushion formation: lessons from mice and chicken. Differentiation 84(1):89–102
Lage K, Greenway SC, Rosenfeld JA, Wakimoto H, Gorham JM, Segre AV et al (2012) Genetic and environmental risk factors in congenital heart disease functionally converge in protein networks driving heart development. Proc Natl Acad Sci USA 109(35):14035–14040
Langlois D, Hneino M, Bouazza L, Parlakian A, Sasaki T, Bricca G et al (2010) Conditional inactivation of TGF-beta type II receptor in smooth muscle cells and epicardium causes lethal aortic and cardiac defects. Transgenic Res 19(6):1069–1082
Lindinger A, Schwedler G, Hense HW (2010) Prevalence of congenital heart defects in newborns in Germany: results of the first registration year of the PAN Study (July 2006–June 2007). Klin Padiatr 222(5):321–326
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V et al (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2095–2128
Mantel PY, Schmidt-Weber CB (2011) Transforming growth factor-beta: recent advances on its role in immune tolerance. Methods Mol Biol 677:303–338
Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M (2014) Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 130(9):749–756
Martin M, Rodriguez I, Palacin M, Rios-Gomez E, Coto E (2011) TGFBR2 gene mutational spectrum in aortic pathology. J Am Coll Cardiol 57(4):518–519 author reply 519
Massague J (2012) TGFbeta signalling in context. Nat Rev Mol Cell Biol 13(10):616–630
Massague J, Blain SW, Lo RS (2000) TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103(2):295–309
Matyas G, Arnold E, Carrel T, Baumgartner D, Boileau C, Berger W et al (2006) Identification and in silico analyses of novel TGFBR1 and TGFBR2 mutations in Marfan syndrome-related disorders. Hum Mutat 27(8):760–769
Mercado-Pimentel ME, Runyan RB (2007) Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs 185(1–3):146–156
Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, Morisaki T et al (2004) Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet 36(8):855–860
Nakajima Y, Yamagishi T, Hokari S, Nakamura H (2000) Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP). Anat Rec 258(2):119–127
Patel SS, Burns TL, Botto LD, Riehle-Colarusso TJ, Lin AE, Shaw GM et al (2012) Analysis of selected maternal exposures and non-syndromic atrioventricular septal defects in the National Birth Defects Prevention Study, 1997–2005. Am J Med Genet A 158A(10):2447–2455
Penny DJ, Vick GW 3rd (2011) Ventricular septal defect. Lancet 377(9771):1103–1112
Person AD, Klewer SE, Runyan RB (2005) Cell biology of cardiac cushion development. Int Rev Cytol 243:287–335
Robson A, Allinson KR, Anderson RH, Henderson DJ, Arthur HM (2010) The TGFbeta type II receptor plays a critical role in the endothelial cells during cardiac development. Dev Dyn 239(9):2435–2442
Rodriguez FH 3rd, Moodie DS, Parekh DR, Franklin WJ, Morales DL, Zafar F et al (2011) Outcomes of hospitalization in adults in the United States with atrial septal defect, ventricular septal defect, and atrioventricular septal defect. Am J Cardiol 108(2):290–293
Santibanez JF, Quintanilla M, Bernabeu C (2011) TGF-beta/TGF-beta receptor system and its role in physiological and pathological conditions. Clin Sci 121(6):233–251
Savagner P (2010) The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol 21(Suppl 7):vii89–vii92
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113(6):685–700
Stevens MV, Broka DM, Parker P, Rogowitz E, Vaillancourt RR, Camenisch TD (2008) MEKK3 initiates transforming growth factor beta 2-dependent epithelial-to-mesenchymal transition during endocardial cushion morphogenesis. Circ Res 103(12):1430–1440
Takenoshita S, Hagiwara K, Nagashima M, Gemma A, Bennett WP, Harris CC (1996) The genomic structure of the gene encoding the human transforming growth factor beta type II receptor (TGF-beta RII). Genomics 36(2):341–344
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890
Townsend TA, Robinson JY, How T, DeLaughter DM, Blobe GC, Barnett JV (2012) Endocardial cell epithelial-mesenchymal transformation requires Type III TGFbeta receptor interaction with GIPC. Cell Signal 24(1):247–256
van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ (2011) The changing epidemiology of congenital heart disease. Nat Rev Cardiol 8(1):50–60
van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ et al (2011) Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 58(21):2241–2247
Wang J, Luo XJ, Xin YF, Liu Y, Liu ZM, Wang Q et al (2012) Novel GATA6 mutations associated with congenital ventricular septal defect or tetralogy of fallot. DNA Cell Biol 31(11):1610–1617
Wik E, Raeder MB, Krakstad C, Trovik J, Birkeland E, Hoivik EA et al (2013) Lack of estrogen receptor-alpha is associated with epithelial-mesenchymal transition and PI3K alterations in endometrial carcinoma. Clin Cancer Res 19(5):1094–1105
Xie J, Chen Y, Li H, Zhou B, Rao L (2012) Association between rs6658835 polymorphism of transforming growth factor beta 2 gene and congenital heart diseases in Chinese Han population. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 29(2):210–213
Yamagishi T, Ando K, Nakamura H (2009) Roles of TGFbeta and BMP during valvulo-septal endocardial cushion formation. Anat Sci Int 84(3):77–87
Yao G, Yin M, Lian J, Tian H, Liu L, Li X et al (2010) MicroRNA-224 is involved in transforming growth factor-beta-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol Endocrinol 24(3):540–551
Zhu X, Deng X, Huang G, Wang J, Yang J, Chen S et al (2014) A novel mutation of Hyaluronan synthase 2 gene in Chinese children with ventricular septal defect. PLoS One 9(2):e87437
Acknowledgments
We would like to acknowledge the helpful comments on this paper received from our reviewers.
Conflict of interest
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiang-Ting Li and Chang-Qing Shen are first co-authors.
Rights and permissions
About this article
Cite this article
Li, XT., Shen, CQ., Zhang, R. et al. Association of TGFBR2 rs6785358 Polymorphism with Increased Risk of Congenital Ventricular Septal Defect in a Chinese Population. Pediatr Cardiol 36, 1476–1482 (2015). https://doi.org/10.1007/s00246-015-1189-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-015-1189-2