Abstract
The arterial switch operation (ASO) remains a challenging procedure, especially in cases with a complicated coronary anatomy. In recent years, the autologous flap extension technique has been used for coronary implantation in complicated ASOs. Operative techniques and indications are discussed in this report. From January 2006 to June 2011, ASO with the autologous flap extension technique for coronary implantation was used for 21 patients, including five cases involving transposition of the great arteries (TGA) with an intact ventricular septum, eight cases involving TGA with a ventriculoseptal defect, five cases of Taussig–Bing anomaly, and three cases involving corrected TGA. Age at operation varied between 3 and 314 days (median, 110 days). Body weight varied between 3.1 and 14 kg (median, 5.4 kg). Three patients underwent a two-stage operation. In all the patients, the main trunk of the right coronary artery or the dilated right ventricular conus branch originated from the left- or right-facing sinus and followed an abnormal course of anterior looping to the aorta. The operative techniques included a long coronary button excised from the aorta and a pedicle flap on the pulmonary artery (neoaorta) cut as a cuff extended to the button of the coronary artery with equal distance. The side edges of the flap and the button were sutured to each other, thus forming an extension tube that lengthened the coronary artery. No in-hospital operative mortality occurred. Delayed sternum closure occurred in five cases. The average mechanical ventilator time was 101.6 h. The average intensive care unit stay was 9.5 days. Follow-up evaluation after discharge was complete in 17 cases. Growth and development were improved in all patients. No ischemic electrocardiographic changes occurred. One patient underwent reoperation for supravalvular pulmonary stenosis 2 years later. The autologous flap extension technique for coronary implantation in complicated ASOs can decrease hospital mortality due to abnormal coronary arteries, especially for patients undergoing two-stage ASOs or patients whose main trunk of the right coronary artery or dilated right ventricular conus branch originates from the left- or right-facing sinus and follows an abnormal course of anterior looping to the aorta.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The arterial switch operation (ASO) has become the procedure of choice for correcting transposition of the great arteries (TGA) and Taussig–Bing anomaly. With the improved technical aspects of the procedure in the last 30 years, the operative mortality associated with this procedure has decreased remarkably [2, 3, 11, 19].
The critical point in the ASO is the successful reimplantation of the coronary arteries. Certain anatomic variations of the coronary arteries, such as a single coronary orifice or an intramural coronary artery, still are considered increased surgical risks because of tension, torsion, or kinking of the coronary arteries. Even mild obstruction of the coronary arteries may lead to early death after the operation. Transfer of the coronary arteries especially remains difficult for neonates and infants. Over the past 5 years, we have performed ASO with the autologous flap extension technique for reimplantation of coronary arteries in patients with complicated malformation of the coronary arteries and have obtained satisfactory operative outcomes.
Materials and Methods
Approval for this retrospective study was obtained from the Shanghai Children’s Medical Center and the affiliated Medical College of Jiao Tong University of Medicine Human Investigation Committee.
Patients and Clinical Presentation
From January 2006 to June 2011, the ASO with the autologous flap extension technique for coronary artery reimplantation was used for 21 consecutive patients (13 boys and eight girls) by one surgeon. Five cases involved TGA with an intact ventricular septum (TGA/IVS). The age at the operation varied from 3 to 65 days (median, 21.4 ± 25.6 days). The weight ranged from 3.1 to 4.8 kg (median, 3.7 ± 0.73 kg). The mean preoperative oxygen saturation was 68.4 ± 5.94 % (range, 60–75 %).
Three patients had concurrent patent ductus arteriosus and atrial septal defect (ASD). Two patients, a 3-day-old infant with a patent foramen ovale and a 4-day-old infant with an intact septal ventricular septum, underwent an emergency operation because of severe hypoxia and consequent circulatory failure.
One 65-day-old infant with obvious shift of the interventricular septum to the left and a 31 g/m2 mass index of the left ventricular myocardium underwent a two-stage ASO. The first-stage operation included pulmonary artery banding, a modified Blalock-Taussig shunt, and ASD enlargement. When the mass index of the left ventricular myocardium was elevated to 65 g/m2 2 weeks later, the second-stage operation was performed.
Eight cases involved TGA with a ventriculoseptal defect (TGA/VSD). The ages of these patients at surgery varied from 35 to 180 days (median, 86.3 ± 55.9 days), and their mean weight was 4.5 ± 1.01 kg (range, 3.4–6.5 kg). Their mean preoperative oxygen saturation was 77.5 ± 3.82 % (range, 72–83 %). Two of the patients had associated coarctation of the aorta. All the patients experienced cardiac insufficiency in various degrees including hepatomegaly, tachypnea, and tachyarrhythmia. One patient underwent urgent tracheal intubation and surgical repair within 48 h after admission because of acute heart failure and pulmonary infection.
Taussig–Bing anomaly was diagnosed in five cases. Their ages at surgery varied from 33 to 314 days (median, 121.2 ± 116.5 days), and their mean weight was 5.2 ± 1.50 kg (range, 4.5–7.5 kg). Their mean preoperative oxygen saturation was 78.0 ± 4.95 % (range, 71–84 %). All these patients underwent a selective ASO.
Corrected TGA (L-TGA) was diagnosed for three patients. Their ages varied from 3 months to 1 year (mean, 213.0 ± 141.4 days), and their mean weight was 8.1 ± 5.2 kg (range, 4.7–14 kg). The preoperative oxygen saturation of each patient exceeded 90 %. One patient with concurrent VSD and pulmonary hypertension underwent a one-stage, double-switch operation. Two other infants with IVS were respectively 6 months and 1 year of age. They underwent a two-stage operation due to degeneration of left heart function, a shift of the interventricular septum to the left, and moderate to severe tricuspid valve regurgitation. The first-stage operation was pulmonary artery banding to maintain the interventricular septum in the middle position and to reduce tricuspid valve regurgitation to a mild degree. A second-stage double-switch operation was performed a year later.
All the patients had an echocardiographic examination, and 17 patients had an enhanced computed tomography (CT) scan or magnetic resonance examination. Seven patients underwent cardiac catheterization examination for a definite diagnosis. The preoperative cardiac anatomy, great artery, and coronary artery patterns are classified in Table 1.
Surgical Management
The patients underwent ASO through a median sternotomy incision. The pericardium was harvested. The ascending aorta appeared slender, and the pulmonary artery was thickset.
After systemic heparinization, cardiopulmonary bypass was established by cannulation of the aorta and the right atrial appendage. Surgery was performed on the cardiopulmonary bypass at high flow (150–170 mL/(kg min)) with a rectal temperature of 25 to 27 °C. Myocardial protection was achieved using cold-blood anterograde cardioplegia. Before cardiac arrest, the great arteries were dissected, and both the right and left pulmonary arteries were widely mobilized for the later Lecompte maneuver. The ventricular and ASDs and the patent ductus arteriosus were completely closed. The VSD was closed with a patch of autologous pericardium.
The coronary artery anatomy was determined according to the description available in the operative reports. The various coronary patterns are summarized in Fig. 1a–e. In our cases, the main trunk of the right coronary artery or the dilated right ventricular conus branch originated from the left- or right-facing sinus and followed an abnormal course of anterior looping to the aortic root and right ventricular outlet tract. This anterior looping resulted in a greater distance between the neocoronary orifice and the distal coronary button, with the potential for stretching of the transferred coronary artery, or bow-stringing, as it crossed the neopulmonary root. We therefore used the autologous flap extension technique in coronary implantations.
a Left anterior descending coronary artery (LAD), left circumflex coronary artery (LCX), and right conus branch (RCB) arising from sinus 1; right coronary artery (R) arising from sinus 2 (n = 7), b LAD and LCX arising from sinus 1; R and RCB arising from sinus 2 (n = 2), c LAD and R arising from sinus 1; LCX arising from sinus 2 (n = 8), d A single sinus coronary artery with one orifice; LAD, LCX, and R arising from sinus 1 (n = 2), e A single sinus coronary artery with two orifices and two coronary arteries with two orifices but in the same sinus (n = 2)
The ascending aorta was transected at a higher level, more than 10 mm above the origin of the coronary artery. Because this incision level was higher than the standard incision in ASO with a normal coronary artery anatomy, the ostium of the anomalous coronary artery could be excised with a longer and more generous U-shaped aortic cuff (coronary button). The pulmonary trunk was divided just proximal to its bifurcation so more distance could be contained.
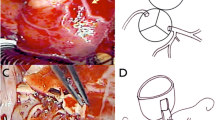
Before the neoaortic reconstruction, a wide reverse U-shaped incision was created in the anterior portion of the neoaorta on the appropriate site so that a pedicle flap on the pulmonary artery (neoaorta) was cut as a cuff and extended to the ostium of the coronary artery with an equal distance for coronary artery implantation. The neoaortic flap tip was sutured around the ostium of the coronary artery. The long coronary button was turned backward. The side edge of this pedicle flap on the pulmonary artery (neoaorta) and the long coronary button previously excised were anastomosed to each other with a 7–0 Prolene suture to form an extension tunnel between the coronary ostium and the neoaortic implantation site that lengthened the coronary artery. The other coronary artery button was reimplanted in a standard manner. A total of 19 pedicle flaps were sutured, with coronary buttons cut from the left coronary sinus. Two were sutured with coronary buttons cut from the right coronary sinus (Fig. 2a–d).
a A long and generous U-shaped aortic cuff (coronary button) was excised, b A wide reverse U-shaped incision was created in the anterior portion of the neoaorta on the appropriate site so that a pedicle flap on the pulmonary artery (neoaorta) could be cut as a cuff and extended to the button of the coronary artery with an equal distance for coronary artery implantation, c The neoaortic flap tip was sutured around the ostium of the coronary artery, d The long coronary button was turned backward. The extension tunnel between the coronary ostium and the neoaortic implantation site was created by suturing both flaps together to lengthen the coronary artery
With the aortic clamp removed, the neopulmonary trunk was repaired with a generous pantaloon-shaped, untreated autologous pericardium patch, and the neopulmonary anastomosis was completed after the Lecompte maneuver. The remainder of the operation was conducted in the usual manner. The sternum was left open if cardiac function was not adequate. Delayed sternal closure was performed 48 to 72 h after hemodynamic stability was achieved. Two patients who had L-loop (corrected) TGA with VSD underwent a double-switch procedure (Senning + ASO).
Postoperative Treatment
Low doses of intravenous dopamine (5.0–7.5 μg/(kg min)) and milrinone (0.5–0.75 μg/(kg min)) were routinely used postoperatively. Patients with low cardiac output obtained additional inotropic medication including dobutamine, epinephrine, and isoprenaline, either alone or in combination. Patients also were sedated and paralyzed during the first 24 to 48 h. Central venous pressure and electrocardiography (ECG) were monitored routinely, and the pulmonary artery pressure (PAP) was monitored for patients older than 6 months. Patients were routinely started on a regimen of sodium nitroprusside (0.5 μg/(kg min)) to reduce afterload and ST changes. Patients with PAP were treated with hyperventilation, sedation, phenoxybenzamine, sildenafil, and inhaled nitric oxide (10–15 ppm) as required.
Results
No operative deaths occurred, and all the patients were discharged from the hospital. The mean cardiopulmonary bypass time was 139.2 ± 42.8 min, and the mean aortic cross-clamp time was 94.1 ± 19.5 min. Left ventricular assist devices and extracorporeal membrane oxygenation were not required in this study group.
Postoperatively, all the patients were sedated and paralyzed during the first 24 to 48 h, and the mean duration of ventilation was 101.6 ± 53.6 h. The median cardiac intensive care unit time was 9.5 ± 4.9 days. Delayed sternal closure was required for five patients 72 h after achievement of stable hemodynamics. Nine patients with low cardiac output received additional inotropic medication including dobutamine, epinephrine, and isoprenaline, either alone or in combination.
A paroxysmal pulmonary hypertensive crisis developed for two patients and was controlled with a combination of inhaled nitric oxide (10–15 ppm), phenoxybenzamine, and sildenafil citrate. Six patients recovered from postoperative pulmonary infection. Low cardiac output with renal insufficiency led to peritoneal dialysis for two patients.
Follow-up evaluation was completed for 17 operative survivors. The follow-up period was 2 months to 3.5 years (mean, 16.8 ± 9.1 months). Interval ECG, chest x-ray, and echocardiogram constituted the follow-up study of choice, and coronary angiography was not routinely performed. There was no ECG evidence suggesting myocardial ischemia in any of the surviving patients. The cardiothoracic ratio of survivors decreased significantly with a normal pulmonary blood supply. All the survivors were categorized as New York Heart Association (NYHA) functional class 1, without medication and in normal sinus rhythm.
Only one patient had right ventricular outflow tract obstruction (echocardiographic gradient, >30 mmHg). He underwent reoperation 2 years later. Doppler studies showed no obvious subaortic obstruction (gradient at rest, <30 mmHg) in any patient. Neoaortic incompetence was moderate in one patient and trivial or mild in two additional patients. No significant pulmonary valvular regurgitation was observed in any patient.
Figure 3a, b shows that the tunnel of the left coronary artery was patent in one TGA/IVS patient 3 years after surgery. Postoperative CT coronary angiography was performed for another TGA/IVS patient 1 year after surgery, with successful coronary transfer using the autologous flap extension technique, showing patent coronary arteries (Fig. 4a, b). None of the follow-up patients experienced a late death.
a Postoperative computed tomographic (CT) coronary angiography of one patient showing the anteroposterior relationship of the great arteries after the Lecompte maneuver. The left anterior descending coronary artery (LAD), the left circumflex coronary artery (LCX), and the right conus branch (RCB) arise from the left-side tunnel of the neoaorta with the anterior looping right conus branch (RCB). PA, pulmonary artery; AO, aorta, b The right coronary artery arising from the right side of the neoaorta was slender, perhaps because of the existing thick RCB. RCA, right coronary artery
Discussion
The ASO has become the most commonly used procedure of choice for a Taussig–Bing double-outlet right ventricle (DORV) and other types of dextro-TGA. The most critical step in ASO is reimplantation of the coronary arteries. Despite advancement in the technical aspects of the procedure, certain anatomic variations of the coronary arteries, such as a single coronary orifice or intramural coronary artery, still are considered surgical risks.
Optimizing the surgical technique for relocating these challenging variations of the coronary anatomy is critical to improving the surgical outcomes of the procedure [5, 9, 10, 17]. Various technical modifications concerning translocation of the complex coronary arteries have been introduced over the past 20 years, with low mortality and morbidity rates. Some authors have introduced an effective modification of the “bay window” technique to allow safer transfer in patients with a short main coronary trunk or for great vessels that are side by side [18]. Other procedures, including modified coronary button, pericardial hood, and in situ coronary relocation techniques, also have been described for dealing with tension, torsion, or kinking of the coronary arteries in ASO patients with complex coronary configurations such as abnormal ostia and an intramural coronary artery [7, 14].
In 1988, Planché et al. [8] described a variety of techniques for various coronary artery patterns. Different coronary artery patterns require appropriate techniques for translocation of the coronary artery to the neoaorta. A looping course is defined as one or more of the three main coronary arteries following an abnormal course in front of the great arteries or behind them. Posterior looping refers to one of the major arteries running posterior to the pulmonary trunk, whereas anterior looping refers to an abnormal course anterior to the aorta.
Posterior looping results in a shorter distance and an acute turn between the neocoronary orifice and the coronary artery, thus posing a risk for kinking or distortion of the proximal segment of the transferred coronary artery. In most instances of posterior looping, transfer of the coronary artery at a higher position than usual helps to avoid kinking.
Conversely, anterior looping results in a greater distance between the neocoronary orifice and the distal coronary segment, with the potential for stretching of the transferred coronary artery as it crosses the neopulmonary root. Stretching of the coronary artery with anterior looping during the transfer can be reduced by placing the coronary button at a lower position [6]. We have found that delicate tissues of the pulmonary artery sinuses are subject to tearing and frequent bleeding in the course of anastomosis.
Scheule et al. [12] conducted a retrospective analysis of 53 patients with a single coronary artery who underwent the ASO between 1983 and 2000 at Children’s Hospital Boston. They found that the survival rate for all the patients was 91 % at 6 months and 87 % at 5 and 10 years after the ASO. The survival rates and freedom from reintervention were lower for the patients who had a single right ostium with the left main coronary artery posterior to the pulmonary artery than for all the other subtypes [12].
Certain coronary patterns in our study included 1LAD,LCX,RCB2R, 1LAD,LCX2R RCB, 1LAD,R2LCX, 1R,LAD,LCX, and 1R1LAD,LCX, with coronary arteries looped anterior to the aorta without exception. These included the right coronary artery with a single left ostium looped anterior to the aorta in four patients (1R,LAD,LCX, 1R1LAD,LCX), the right coronary artery simple looped anterior to the aorta in eight patients (1LAD,R 2LCX), and the dilated right ventricular conus branch looped anterior to the aorta in nine patients.
Reimplantation using a conventional coronary transfer technique could cause kinking or overstretching of coronary arteries at their origin during rotation of the coronary button because of the excessively long distance. A higher incidence of an intramural course of the coronary artery adds to these difficulties. We have managed these complex coronary arteries with the autologous flap extension technique.
The idea for the autologous flap extension technique has come from procedures used in the surgical treatment for an anomalous origin of the left coronary artery from the pulmonary trunk (ALCAPA) [1, 13]. In 2011, Turkoz et al. [16] described a similar technique for coronary artery reimplantation using a pedicle flap on the pulmonary artery to create a tunnel resulting in an extension of the coronary button. They considered that this technique was suitable for anomalies in which the reimplantation site was distant in older patients and the dissection of the coronary artery was limited. This technique increased the distance between the coronary ostium and the reimplantation site and prevented stretching of the coronary artery by providing an extra 0.5 to 1 cm at the anastomosis site even when the heart distended beyond normal size postoperatively. Furthermore, with this technique, minor angulation of the coronary artery may be better tolerated during reimplantation due to less tension. Myocardial ischemia due to stretching of the coronary artery after reimplantation was greatly reduced in patients with anterior looping or distant reimplantation distance.
Three patients in our group underwent two-stage ASO, and two of these patients had L-loop (corrected) TGA with VSD and underwent a double-switch procedure. All the patients had a coronary anomaly, defined as the right ventricular conus branch arising from the left sinus. The two-stage operation usually causes coronary arterial orifices and branches adherent to the surrounding tissues and overmobilizing synechia, with the potential for irreversible damage to the coronary arteries. However, with the autologous flap extension technique, we try to keep the right ventricular conus branch intact.
Because the sinuses of the pulmonary trunk usually were relatively wider in most patients, it was necessary to mobilize the posterior valvular commissure from the pulmonary artery wall according to the implantation distance before suturing the coronary button. Therefore, an unobstructed extension tube between the coronary ostium and the neoaortic implantation site could be achieved. Furthermore, because the coronary tube constructed by autologous tissues reserves the capacity for growth in neonates and infants, calcification risk could be avoided in the future [15].
References
Alexi-Meskishvili V, Nasseri BA, Nordmeyer S, Schmitt B, Weng YG, Böttcher W et al (2011) Repair of anomalous origin of the left coronary artery from the pulmonary artery in infants and children. J Thorac Cardiovasc Surg 142:868–874
Alsoufi B, Cai S, Williams WG, Coles JG, Caldarone CA, Redington AM et al (2008) Improved results with single-stage total correction of Taussig-Bing anomaly. Eur J Cardiothorac Surg 33:244–250
Freed DH, Robertson CM, Sauve RS, Joffe AR, Rebeyka IM, Ross DB et al (2006) Intermediate-term outcomes of the arterial switch operation for transposition of great arteries in neonates: alive but well? J Thorac Cardiovasc Surg 132:845–852
Gittenberger de Groot AC, Sauer U, Quaegebeur J (1986) Aortic intramural coronary artery in three hearts with transposition of the great arteries. J Thorac Cardiovasc Surg 91:566–571
Gottlieb D, Schwartz ML, Bischoff K, Gauvreau K, Mayer JE (2008) Predictors of outcome of arterial switch operation for complex D-transposition. Ann Thorac Surg 85:1698–1703
Lacour-Gayet F (2007) Arterial switch operation with ventricular septal defect repair and aortic arch reconstruction. Semin Thorac Cardiovasc Surg 19:245–248
Murthy KS, Coelho R, Kulkarni S, Ninan B, Cherian KM (2004) Arterial switch operation with in situ coronary reallocation for transposition of great arteries with single coronary artery. Eur J Cardiothorac Surg 25:246–249
Planché C, Bruniaux J, Lacour-Gayet F, Kachaner J, Binet JP, Sidi D et al (1988) Switch operation for transposition of the great arteries in neonates: a study of 120 patients. J Thorac Cardiovasc Surg 96:354–363
Qamar ZA, Goldberg CS, Devaney EJ, Bove EL, Ohye RG (2007) Current risk factors and outcomes for the arterial switch operation. Ann Thorac Surg 84:871–879
Raisky O, Bergoend E, Agnoletti G, Ou P, Bonnet D, Sidi D et al (2007) Late coronary artery lesions after neonatal arterial switch operation: results of surgical coronary revascularization. Eur J Cardiothorac Surg 31:894–898
Sarris GE, Chatzis AC, Giannopoulos NM, Kirvassilis G, Berggren H, Hazekamp M et al (2006) The arterial switch operation in Europe for transposition of the great arteries: a multi-institutional study from the European Congenital Heart Association. J Thorac Cardiovasc Surg 132:633–639
Scheule AM, Zurakowski D, Blume ED, Stamm C, del Nido PJ, Mayer JE Jr et al (2002) Arterial switch operation with a single coronary artery. J Thorac Cardiovasc Surg 123:1164–1172
Sese A, Imoto Y (1992) New technique in the transfer of an anomalously originated left coronary artery to the aorta. Ann Thorac Surg 53:527–529
Shukla V, Freedom RM, Black MD (2000) Single coronary artery and complete transposition of the great arteries: a technical challenge resolved? Ann Thorac Surg 69:568–571
Takeuchi S, Katogi T (1990) New technique for the arterial switch operation in difficult situation. Ann Thorac Surg 50:1000–1001
Turkoz R, Ayabakan C, Vuran C, Omay O, Tokel K (2011) Extension of coronary artery with double-flap technique in a complicated arterial switch operation. J Card Surg 26:324–327
Ugurlucan M, Sayin OA, Surmen B, Tireli E (2006) Coronary reimplantation after neoaortic reconstruction in arterial switch operation. Ann Thorac Surg 82:382
Yamagishi M, Shuntoh K, Fujiwara K, Shinkawa T, Miyazaki T, Kitamura N (2003) “Bay window” technique for the arterial switch operation of the transposition of the great arteries with complex coronary arteries. Ann Thorac Surg 75:1769–1773
Zheng JH, Xu ZW, Liu JF, Su ZK, Ding WX (2008) Arterial switch operation with coronary arteries from a single sinus in infants. J Card Surg 23:606–610
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Xu, Z., Liu, J. et al. Coronary Implantation Using the Autologous Flap Extension Technique in Complicated Arterial Switch Operations. Pediatr Cardiol 34, 795–801 (2013). https://doi.org/10.1007/s00246-012-0535-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-012-0535-x