Abstract
Infants with hypoplastic left heart syndrome (HLHS) represent a high-risk population when they present for noncardiac surgery. To assist clinicians in the care of these infants, we present our experience of 36 HLHS patients who underwent abdominal surgery after stage I palliation. We reviewed patients with HLHS who underwent gastrostomy and/or fundoplication after stage I palliation during an 18-month period. We assessed the impact of preoperative echocardiographic predictors and regional anesthesia on use of intraoperative inotropes, extubation in the OR, perioperative instability, postoperative escalation of care, and length of hospital and intensive care unit stay. Of 39 abdominal operations, all but 2 were performed with open laparotomy. There was a positive association between regional anesthesia and instability during induction. Escalation of respiratory care occurred in 9 (23.1%) cases, and escalation of hemodynamic care occurred in 6 (15.4%) cases. Neoaortic valve insufficiency was associated with increased length of stay, and ventricular outflow obstruction was associated with escalation of hemodynamic care. Extubation in the OR was successful in 31 cases (79.5%). In-hospital death occurred in 1 patient (2.7%). HLHS infants often undergo abdominal surgery, but intraoperative instability and need for escalation of care is common. Specific echocardiographic findings were associated with length of stay and escalation of care. Regional anesthesia was associated with transient intraoperative instability but not with other adverse outcomes.
Similar content being viewed by others
Introduction
Patients with hypoplastic left heart syndrome (HLHS) have a unique physiology that carries significant medical and surgical implications. After stage I palliation (SIP [either Norwood or hybrid procedure]) for HLHS, the right ventricle functions as the systemic ventricle. Pulmonary blood flow is supplied by either a systemic-to-pulmonary shunt, usually a modified Blalock-Taussig shunt (mBTS); a right ventricle-to-pulmonary artery conduit (Sano shunt); or, in the hybrid procedure, a stented ductus arteriosus. The second stage surgery, or stage II palliation (SIIP), occurs several months later and is typically a superior cavopulmonary anastomosis where the pulmonary flow is supplied by the superior vena cava.
The interstage period between SIP and SIIP is associated with significant mortality, with 4% to 15% of infants dying between discharge from the hospital and SIIP [1]. Coronary ischemia, excessive or insufficient pulmonary blood flow, neoaortic obstruction, and systemic ventricle failure are among the top reported causes of death in this population [2]. Patients with single-ventricle physiology respond poorly to decreases in preload and acute increases in afterload [18], both of which are prone to occur in the perioperative setting, especially in cases of abdominal or laparoscopic surgery. For these reasons, minimization of the stress response and maintenance of preload are crucial in the perioperative management of these patients [16].
Infants with HLHS frequently have comorbidities, such as gastroesophageal reflux, oropharyngeal dysfunction, and failure to thrive, which require surgery and general anesthesia, often during the tenuous SIP/SIIP interstage period. Gastrostomy with fundoplication has demonstrated benefit for nutritional deficits and failure to thrive in this population and is one of the more frequently performed procedures [3, 7, 13]. Perioperative management of these infants is challenging because of wide institutional variability and a body of literature on the subject that consists of retrospective case series [3–6, 8, 9, 12, 14, 15, 17]. Here we describe our recent experience of 39 gastrostomy and fundoplication procedures in 36 infants with HLHS after SIP at our institution, where specific preoperative factors are tested for their impact on postoperative outcomes. The results address the question of how to appropriately manage these patients in the perioperative setting.
Methods
After receiving Institutional Review Board approval, we queried the Society of Thoracic Surgeons database for our institution, searching for records bearing procedural code 33619 (Norwood surgery) and diagnostic code 746.7 (hypoplastic left heart syndrome) between July 2006 and December 2009. All but 1 Norwood surgery were performed by the same surgeon. The resulting 64 charts were then reviewed for gastrostomy and/or gastric fundoplication during the study interval, resulting in 36 patients who comprised the study population.
We extracted preoperative, intraoperative, and postoperative data from patient records using the following definitions. Preoperative laboratory tests were defined as the last values assayed on the patient before surgery. Preoperative echocardiogram was defined as present if performed within 1 week before the abdominal procedure, and postoperative echocardiogram was defined as present if performed within 24 h after surgery. Intraoperative or postoperative inotropic drugs were defined as any drugs administered by infusion with the intent to increase cardiac output while the patient was in the operating room (OR) or during the first postoperative 24 h, respectively. Intraoperative hemodynamic instability was defined as the following: (1) measured systolic blood pressure <75% of baseline systolic blood pressure for >10 min or that required pharmacologic support with vasoactive drugs by infusion or two or more doses or fluid bolus >20 ml/kg; (2) systolic blood pressure >25% above baseline for >10 min; (3) heart rate <120 or >200 for >10 min; and (4) any decrease in heart rate that prompted specific pharmacologic treatment, significant rhythm change or major arrhythmia, or arterial saturation <70% or >20% lower than baseline while the patient was in the OR. We chose markers of instability that were measurable by standard American Society of Anesthesiologist (ASA) monitors, which were available for providers at the bedside during most, if not all, administration of anesthetics and could be easily tracked during the perioperative period.
Echocardiographic abnormalities were defined as those stated on the echocardiogram report within 1 week before surgery. Ventricular outflow tract obstruction, either neoaortic valvar stenosis, residual aortic arch gradient, or residual coarctation, was defined as present if listed in the summary on the echocardiographic report and was classified as mild, moderate, or severe. Tricuspid and neoaortic valve insufficiency were defined as present if included on the preoperative echocardiogram report and were classified as mild, moderate, or severe. Right-ventricular wall motion was classified as normal or by degree of dysfunction (mild, moderate, or severe).
Surgical times were defined as follows: Induction was defined as the time from OR entry to skin incision; maintenance was defined as the time from incision to skin closure; and emergence was defined as the time from skin closure to OR exit. Recovery was defined as the time from OR exit to the end of recovery room stay or the first postoperative hour for direct intensive care unit (ICU) admissions. Postoperative transfusion was defined as the administration of red cells or other blood products within 1 day of surgery. Evidence of decreased perfusion was defined as the need for inotropic or vasoactive drug infusion within 24 h of surgery, postoperative increase in blood lactate >2.2, base deficit >–2, acidosis with pH <7.25, or postoperative chest X-ray consistent with pulmonary edema, new infiltrate, or effusion. Escalation of respiratory care was defined as an increase in supplemental oxygen, reintubation, initiation of noninvasive positive pressure ventilation, or invasive ventilation. Escalation of hemodynamic care was defined as the need for initiation or escalation of inotropic/vasoactive infusions, agents to decrease afterload, antihypertensive drugs, or antiarrhythmic drugs. Postoperative laboratory reports were defined as the first laboratory values drawn within 48 h after surgery. Complications were broadly defined as unexpected events that warranted significant escalation of care.
Data are presented as means ± SDs for continuous data or as percent for dichotomous data. Statistical analysis, where performed, involved comparison of outcome measures with specified preoperative and intraoperative variables of interest. Comparisons were performed using SPSS software and Fisher’s exact test for discrete data or Student t test or Mann–Whitney U-test where appropriate for continuous data where comparisons involved unpaired groups. Spearman’s Rho correlation was used for quantifying the association between two continuous variables (p < 0.05).
Results
Study Population
Initial chart screening yielded 64 infants with HLHS who underwent Norwood palliation during the study interval (July 2006–December 2009). Of these 64 subjects, 39 underwent gastrostomy, and 37 of the 39 patients had concomitant Nissen fundoplications. In addition, 2 subjects also had an enterostomy takedown. One patient had an aborted laparoscopic gastrostomy and fundoplication due to hemodynamic instability after abdominal insufflation. That patient underwent open gastrostomy and Nissen fundoplication 1 week later and therefore accounts for 2 cases in the study group.
The population of patients and preoperative parameters studied are listed in Table 1. Most patients underwent stage I palliation during the first week of life. The most common source of pulmonary blood flow was a mBTS (n = 34 [87%]), followed by Sano (n = 3 [7%]), central shunt (n = 1 [2%]), and hybrid palliation with a stented ductus arteriosus (n = 1 [2%]). The average age of patients at the time of noncardiac surgery was 46 days (range 20–137). All subjects were inpatients at the time of abdominal surgery and had an average preoperative length of stay of 38 days (range 2–165). All but 1 had been an inpatient since birth. All but 2 patients underwent an open abdominal procedure. Laparoscopic procedures were attempted on 3 patients, with 1 patient having hemodynamic instability (ventricular bigeminy, hypotension, and bradycardia) on insufflation of the abdomen to 10 mmHg, resulting in the procedure being aborted.
Perioperative Events
Important perioperative events are listed in Table 2. The majority of patients (79.5%) were extubated in the OR. Induction instability occurred in 69.2% of cases. Instability was also common during emergence (48.7%), and recovery (38.5%). Most intraoperative instability, although common, was transient; however, two cases escalated to cardiac arrest, requiring urgent implementation of extracorporeal circulation (ECMO).
Echocardiographic Findings
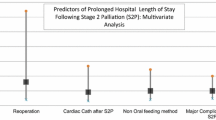
All patients had a preoperative echocardiogram performed within 1 week of surgery. Echocardiographic abnormalities studied were limited to right-ventricular dysfunction (present in 31% of cases), ventricular outflow obstruction (5%), neoaortic valve insufficiency (67%), and tricuspid insufficiency (87%). At least one of these findings was observed in each of the cases. These findings were variously associated with length-of-stay parameters, ventilator time, escalation of care, and emergence instability as listed in Table 3. Ventricular dysfunction was associated with increased total hospital stay (p = 0.014) and ventilator days (p = 0.011; Mann–Whitney U-test). Ventricular outflow obstruction was associated with increased total hospital (p = 0.041) and ICU stay (p = 0.026). In addition, ventricular outflow obstruction was associated with postoperative escalation of hemodynamic care (p = 0.020; Fisher’s exact test). Neoaortic valve insufficiency occurred in 26 patients and was classified as mild in all cases. Neoaortic valve insufficiency was associated with hospital stay (p = 0.010), ICU stay (p = 0.002), and ventilator time (p = 0.005) but was negatively correlated with emergence instability (p = 0.019). Tricuspid insufficiency was the most common echocardiographic anomaly classified and was found in 87% of patients; with 59% having mild, 25% having moderate, and 2% having moderate to severe cases. Neither tricuspid insufficiency nor its severity was associated with any measure of adverse outcome.
Perioperative Management
One third of patients received supplemental oxygen before surgery, including 2 patients who were intubated and mechanically ventilated. Almost all patients underwent intravenous induction of anesthesia (92%). Most patients did not have invasive arterial blood pressure monitoring. Half of the patients received either caudal (16 single shot, 2 continuous catheter) or epidural (1 thoracolumbar) anesthesia in addition to general anesthesia. The decision to provide regional anesthesia was made by the attending anesthesiologist in discussion with the attending surgeon and was based on expectation for postoperative extubation and the anticipated ability of the patient to tolerate a regional technique or systemic analgesics after surgery. Regional anesthesia was contraindicated in many patients due to anticoagulation with enoxaparin (Lovenox). Regional anesthesia was associated with an increased need for intraoperative inotropic drugs (p = 0.018) and instability during the induction phase of anesthesia (p = 0.014, Table 4). Most patients (79.5%) were extubated in the OR. Two of the 8 patients who were not extubated in the OR had been chronically ventilated before surgery. The other 6 had intraoperative hemodynamic instability, including 2 cases of cardiac arrest requiring urgent use of ECMO, which prompted unplanned postoperative transfer to the ICU. There was no significant association between use of regional anesthesia and extubation in the OR; 16 of 19 patients with regional anesthesia, compared with 15 of 20 patients without regional anesthesia, were extubated in the OR (p = 0.695).
Transfusion of blood products, either intraoperatively or within the first 24 h after surgery, was performed in 14 (25.7%) of the 39 cases. Transfusion was associated with an increase in hospital stay (mean 61 vs. 90 days, p = 0.049; Mann–Whitney U test) and a decrease in ventilator-free days in the first 30 postoperative days (28 vs. 23 days, p = 0.002; Mann–Whitney U test).
Postoperative Outcomes
Nine patients (23%) required escalation of postoperative respiratory care as defined previously. Six patients (15.4%) required escalation of hemodynamic care during the postoperative period. Five patients (12.8%) had unplanned admissions to the ICU, two directly from the OR and three from the hospital floor within 36 h after surgery. One of the unplanned ICU admissions was for cardiopulmonary insufficiency requiring reintubation, initiation of milrinone, and mechanical ventilation on the postoperative day 1. Another patient had inadequate analgesia on the night of surgery, resulting in concern for impending respiratory compromise leading to ICU transfer for monitoring. A third patient had respiratory insufficiency thought to be related to opioid administration for poorly controlled pain; this required transfer to the ICU within 12 h after surgery. A fourth patient had hemodynamic instability after induction of anesthesia that responded to appropriate therapy; gastrostomy was completed, and the patient was admitted to the ICU after surgery. The fifth unplanned ICU admission was a patient who became unstable immediately after abdominal insufflation to 10 mmHg, resulting in cancelation of the procedure and admission to the ICU for monitoring. All unplanned ICU transfer cases recovered and were discharged from the hospital. Mortality was low; one patient died within 30 days of surgery from bradycardic arrest on the hospital floor on postoperative day 7.
Of note, during the study period (2009), two unplanned ICU admissions in this population resulted in a change in institutional practice, requiring all HLHS status post-SIP patients to be admitted to the ICU for observation after receiving general anesthesia. The policy was enacted after a multidisciplinary review of adverse events in this patient population after general anesthesia for invasive and diagnostic procedures. The resulting chart review was the impetus for this study.
Discussion
Adequate enteral feeding can be challenging in the HLHS population [7] because gastroesophageal reflux is common, and aspiration may be poorly tolerated. Other gastrointestinal complications, such as necrotizing enterocolitis, are common after SIP, necessitating abdominal procedures during this high-risk period [5].
A series of adverse outcomes in single-ventricle patients after noncardiac surgery prompted us to examine our experience from a recent cohort of 36 patients with HLHS after SIP. This represents a homogenous group of patients with similar congenital heart lesions who underwent the same palliation at the same institution, thus avoiding confounding variables present in mixed populations. We chose to study these patients undergoing gastrostomy and/or fundoplication because these operations are commonly performed in this population at our institution, are moderately invasive procedures, and have been associated with complications. In addition, these procedures lack accepted management consensus guidelines. Our intent was to provide descriptive statistics, identify perioperative risk factors for adverse outcomes, and provide clinically useful information for risk stratification.
We observed that a preoperative echocardiogram is a valuable source of information for perioperative management. Although mild in all cases, neoaortic valve insufficiency occurred in two of three patients and was associated with increased total ICU and ventilator days. This raises concern that patients with neoaortic valve insufficiency of greater severity or worsening severity may be at greater risk for adverse events and outcomes.
Right ventricle dysfunction was found in 1 of 3 patients, with 1 in 10 having moderate dysfunction. Ventricular dysfunction was associated with increased total hospital, ICU, and ventilator days. Tricuspid valve insufficiency was frequent and found in 87% of patients: 59% having mild, 25% having moderate, and 2% having moderate to severe cases. However, this was not associated with increased hospital or ICU stay, ventilator time, or escalation of care. Although far less common (n = 2 subjects), systemic ventricle outflow obstruction (SVOTO) was associated with increased total hospital, ICU and ventilator stays. In addition, SVOTO was associated with the need for postoperative escalation of hemodynamic care, suggesting a possible greater risk association with this lesion. This correlates with results from the Pediatric Perioperative Cardiac Arrest (POCA) study in which 16% of the cases of cardiac arrest in their study population had ventricular outflow obstruction [11].
Perioperative instability was common and occurred in two of three cases at some point during administration of the anesthetic. The majority of instability occurred during the induction and maintenance phases. Although common, instability was transient in most cases. With the exception of neoaortic valve insufficiency and SVOTO, no other preoperative markers were associated with intraoperative instability. Neoaortic valve insufficiency was negatively associated with emergence instability, a finding of unclear significance.
Regional anesthesia, primarily single-shot caudal, was performed in half of cases and was associated with increased induction instability and the need for intraoperative inotropic drugs. Adequate postoperative analgesia in this population undergoing open procedures with a midline upper abdominal incision is clinically relevant. In our study, at least one patient required ICU transfer due to inadequate postoperative pain control, and poor pain control may have contributed to the decompensation of two other patients who required unplanned ICU transfer. Most episodes of instability, primarily hypotension, occurred during the period between induction of anesthesia and surgical incision, i.e., during a period of minimal stimulation. This is often the time when regional blocks are performed. The benefits commonly associated with use of regional anesthesia were not found to be significant in this case series. Regional anesthesia did not correlate with extubation in the OR, unplanned ICU admission, decreased postoperative escalation of care, or decreased lengths of stay. No studies have examined the hemodynamic effects of caudal anesthesia in patients with HLHS undergoing noncardiac surgery. The largest series of patients with congenital heart disease to receive caudal anesthesia found it to be safe and effective, but only 8 of the 220 patients had single-ventricle physiology, and the procedures performed were cardiac operations [10].We believe that regional anesthesia remains a viable option for this patient population pending the knowledge that intraoperative hypotension is common albeit transient. Because this was not a randomized trial, our data do not address the question of whether regional anesthesia should be either used or avoided in this group of patients.
The majority of patients in this series were extubated in the OR. Extubation in the OR was associated with increased hospital- and ventilator-free days after surgery. This would suggest a beneficial effect of extubation in the OR. The impact of extubation in the OR on the immediate postoperative course is less clear. Most postoperative complications were respiratory in origin, and unplanned ICU admissions and transfers were primarily for respiratory decompensation. During the study period, a policy change resulted in all patients being admitted to the ICU directly from the OR; how this impacted the decision to extubate in the OR is unknown.
There were two episodes of intraoperative cardiac arrest, both requiring use of ECMO. These arrests occurred during the maintenance phase of the anesthetic during both an open and a laparoscopic procedure. The exact etiologies of the arrest were unclear, although decreased pulmonary blood flow through mBTS procedure was postulated. Both patients recovered. One patient died 7 days after an uneventful procedure.
Optimization of perioperative care for patients with HLHS is challenging because data comparing methods of management are sparse. Table 5 lists the studies from the past 20 years of HLHS patients undergoing noncardiac surgery, which show the variability in perioperative management. In 2002, Torres et al. reported nationwide mortality data from 1988 to 1997, during which period gastrostomy was the most frequent noncardiac surgery performed on patients with HLHS <2 years of age [15]. In this report, noncardiac surgery carried a mortality rate (assessed by survival to discharge) of 19%. A 2008 single center review by Cribbs et al. [3] reported 5-year mortality rate of 40.5% in their single-ventricle population after fundoplication, which was no different from the mortality in a cohort of single-ventricle patients not receiving fundoplication. Recent data from the POCA registry has identified patients with single-ventricle lesions, especially those before SIIP, as being the most common congenital heart defects associated with cardiac arrest [11]. In their study, the majority of cardiac arrests in patients with congenital heart disease occurred in general ORs (54%), and the most frequent procedures were gastrointestinal: fundoplication, gastrostomy, esophagogastroduodenoscopy, and colostomy (13%). In the present study, we contribute to this literature by presenting an approach to gastrostomy and antireflux management consisting mostly of open laparotomy, without invasive monitoring, with most patients being extubated in the OR. In addition, this is the largest series of cases of regional anesthesia in patients with HLHS after SIP to be reported in the literature.
Our institutional approach has primarily been to use open technique for gastrostomy and fundoplication in this group of patients because we have been concerned about the potentially harmful hemodynamic effects of increased intra-abdominal pressure and increased PaCO2 on this fragile population of infants. Nevertheless, the merits of the laparoscopic approach, which include less postoperative pain, decreased postoperative need for narcotics, and faster recovery, have led other institutions to use the laparoscopic approach with modifications to address the physiologic consequences of the pneumoperitoneum. Three recent studies [8, 12, 19] reported zero short-term mortality in a total of 19 HLHS patients undergoing laparoscopic surgery (Table 5). These investigators described protocols that include postoperative echocardiography [12], invasive blood pressure monitoring [8, 12, 19], limitation of insufflation pressure [8, 12, 19], routine transfusion for patients with hematocrits of 40% [8] or 45% [12], and planned postoperative ICU admission [3, 8]. Although the physiologic perturbations of laparoscopy have the capacity to decrease cardiac output and systemic-pulmonary shunt flow, possibly resulting in thrombosis, Slater et al. [12] reported no observations of shunt thrombosis by postoperative echocardiography in their series of 12 HLHS infants undergoing laparoscopy.
Limitations
This study has limitations inherent to any retrospective review in that it does not involve a prospective comparison with patients managed by differing approaches. In addition, the subset of HLHS patients presenting for elective abdominal operations is not necessarily representative of the HLHS population as a whole at our institution. During the study interval at our institution, there were 30 patients with HLHS receiving SIP who did not undergo fundoplication and/or gastrostomy; these patients were not part of the study population. Mortality in this group was 67%, with most of these deaths occurring within 2 weeks of surgery. Therefore, the study population represents a subset of HLHS patients who survived the immediate postoperative period. Because this study reported only retrospective data, we are not able to conclude whether early gastrostomy is beneficial in this population. A randomized prospective trial would be the best way to address this question. In addition, because all patients survived the procedure with minimal complications, our data do not allow us to identify a subgroup of patients who may not be candidates for gastrostomy and fundoplication.
The applicability to other institutions and practices is also limited because of differing management techniques, varied decision-making practices, and our small study group. Applicability to other patients with single-ventricle physiology, such as tricuspid atresia or complex heterotaxy, is also limited. Only three of our patients underwent laparoscopic procedures, which makes it difficult to compare the risks and benefits of open laparotomy versus laparoscopy in this population. Finally, the limited sample size makes it difficult to accrue sufficient statistical power to provide a robust regression analysis of multiple risk factors and their interactions.
Conclusion
In summary, we report the management of open gastrostomy and fundoplication in HLHS infants after SIP with low perioperative mortality. We present one of the largest series of this patient population to undergo regional anesthesia for noncardiac surgery. Based on our observations, we can recommend an approach that involves anticipation of perioperative instability, postoperative pain management, and need for escalation of hemodynamic or respiratory care. Invasive monitoring may be part of such preparation. Preoperative echocardiography may be useful in assigning perioperative risk because patients with ventricular dysfunction, outflow tract obstruction, or neoaortic valve insufficiency may be at risk for longer hospital stay and perioperative escalation of care. The need for escalation of respiratory or hemodynamic care is common, and inadequate pain management may precipitate unplanned ICU admission. The common occurrence of intraoperative instability and unplanned ICU admission underscores the need for appropriate resources and experience.
References
Al-Akhfash AA, Kabbani MS, Abu-Sulaiman RM, Tamimi OR, Elbarbary MA, Najm HK (2009) Outcome of Norwood and Damus-Kaye-Stansel procedures for univentricular congenital heart anomalies. Saudi Med J 30:340–345
Bartram U, Grunenfelder J, Van Praagh R (1997) Causes of death after the modified Norwood procedure: a study of 122 postmortem cases. Ann Thorac Surg 64:1795–1802
Cribbs RK, Heiss KF, Clabby ML, Wulkan ML (2008) Gastric fundoplication is effective in promoting weight gain in children with severe congenital heart defects. J Pediatr Surg 43:283–289
Hardy C (2008) Pyloromyotomy in an infant with hypoplastic left heart syndrome status-post hybrid procedure: not just another case? Paediatr Anaesth 18:993–994
Jeffries HE, Wells WJ, Starnes VA, Wetzel RC, Moromisato DY (2006) Gastrointestinal morbidity after Norwood palliation for hypoplastic left heart syndrome. Ann Thorac Surg 81:927–982
Karl HW, Hensley FA Jr, Cyran SE, Frankel CA, Myers JL (1990) Hypoplastic left heart syndrome: anesthesia for elective noncardiac surgery. Anesthesiology 72:753–757
Kelleher DK, Laussen P, Teixeira-Pinto A, Duggan C (2006) Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition 22:237–244
Mariano ER, Boltz MG, Albanese CT, Abrajano CT, Ramamoorthy C (2005) Anesthetic management of infants with palliated hypoplastic left heart syndrome undergoing laparoscopic Nissen fundoplication. Anesth Analg 100:161–1631
Nicolson SC, Steven JM, Kurth CD, Krucylak CP, Jobes DR (1994) Anesthesia for noncardiac surgery in infants with hypoplastic left heart syndrome following hemi-Fontan operation. J Cardiothorac Vasc Anesth 8(3):334–346
Peterson KL, DeCampli WM, Pike NA, Robbins RC, Reitz BA (2000) A report of two hundred twenty cases of regional anesthesia in pediatric cardiac surgery. Anesth Analg 90:1014–1049
Ramamoorthy C, Haberkern CM, Bhananker SM, Domino KB, Posner KL, Campos JS et al (2010) Anesthesia-related cardiac arrest in children with heart disease: data from the pediatric perioperative cardiac arrest (POCA) registry. Anesth Analg 110:1376–1382
Slater B, Rangel S, Ramamoorthy C, Abrajano C, Albanese CT (2007) Outcomes after laparoscopic surgery in neonates with hypoplastic heart left heart syndrome. J Pediatr Surg 42:1118–1121
Srinivasan C, Sachdeva R, Morrow WR, Gossett J, Chipman CW, Imamura M et al (2009) Standardized management improves outcomes after the Norwood procedure. Congenit Heart Dis 4:329–337
Testa L, Tobias JD, Kavanaugh-McHugh A (1994) Hypoplastic left heart syndrome: anesthetic care prior to transplantation or surgical palliation. J Clin Anesth 6:500–504
Torres A Jr, DiLiberti J, Pearl RH, Wohrley J, Raff GW, Bysani GK et al (2002) Noncardiac surgery in children with hypoplastic left heart syndrome. J Pediatr Surg 37:1399–1403
Walker SG, Stuth EA (2004) Single-ventricle physiology: perioperative implications. Semin Pediatr Surg 13:188–202
Walker A, Stokes M, Moriarty A (2009) Anesthesia for major general surgery in neonates with complex cardiac defects. Paediatr Anaesth 19:119–125
Wright GE, Crowley DC, Charpie JR, Ohye RG, Bove EL, Kulik TJ (2004) High systemic vascular resistance and sudden cardiovascular collapse in recovering Norwood patients. Ann Thorac Surg 77:48–52
Wulkan ML, Vasudevan SA (2001) Is end-tidal CO2 an accurate measure of arterial CO2 during laparoscopic procedures in children and neonates with cyanotic congenital heart disease? J Pediatr Surg 36:1234–1236
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watkins, S., Morrow, S.E., McNew, B.S. et al. Perioperative Management of Infants Undergoing Fundoplication and Gastrostomy After Stage I Palliation of Hypoplastic Left Heart Syndrome. Pediatr Cardiol 33, 697–704 (2012). https://doi.org/10.1007/s00246-012-0197-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-012-0197-8