Abstract
Background
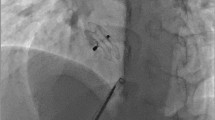
A variety of transcatheter atrial septal defect (ASD) occluders are currently in use, the most commonly used device is the Amplatzer Septal Occluder (ASO) yet there is no perfect device. The Helex Septal Occluder is a new device (by W.L. Gore & Associates, Inc.) designed to improve the results of transcatheter ASD closure. We report our first experience in closing secundum ASDs with this new device after its recent modifications.
Methods
Thirteen patients were selected for Helex device closure with median age of 8 years (2.5–44 years) and median weight 30 Kg (12–96 Kg). Inclusion criteria were: small to moderate Secundum ASDs with sufficient rims by transthoracic echo (TTE) and confirmed by transoesophageal echo (TEE). Two cardiologists carried out the decision of device size. One of the five available Helex ASD device sizes was used (15–35 mm). Follow up TTE was performed the next day, one month, six months and one year later.
Results
Twelve patients had successful Helex septal occluder implantation. One patient was switched to Cribriform ASD device during the procedure because of failure of the locking mechanism. Two patients had trivial residual left to right shunt which disappeared at one month follow up in one patient. No embolic event, AV block or mortality was observed.
Conclusion
This feasibility study of the novel Helex septal occluder after its recent modification showed that it can successfully and safely close well selected secundum ASDs. There are several advantages over the currently available devices.



Similar content being viewed by others
References
Butera G, Carminati M, et al. (2004) CardioSEAL/STARflex versus Amplatzer devices for percutaneous closure of small to moderate (up to 18 mm) atrial septal defects. Am Heart J 148:507–510
Carminati M, Butera G, et al. (2002) Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol 39:1061–1065
Chessa M, Carminati M, Butera G, et al. (2002) Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol 39:1061–1065
Cowley CG, Lloyd TR, Bove EL, et al. (2001) Comparison of results of closure of secundum atrial septal defect by surgery versus Amplatzer septal occluder. Am J Cardiol 88:589–591
Dhilon R, Josen M, Henien M, et al. (2002) Transcatheter closure of atrial septal defect preserves right ventricular. Heart 87:461–465
Du ZD, Hijazi ZM, Kleinman CS, et al. (2002) Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicentre nonrandomized trial. J Am Coll Cardiol 39:1936–1944
Hausdorf G, Kaulitz R, Paul T, et al. (1999) Transcatheter closure of atrial septal defect with a new flexible, self-centering device (the STARFlex Occluder). Am J Cardiol 84:1113–1116
Latson L, Jones T, Zahn E, Rhodes J (2006) Analysis of factors related to successful transcatheter closure of secundum atrial septal defects using the Helex septal occluder. Am Heart J 151:1136e7–1136e11
McMahon CJ, Feltes TF, Fraley JK, et al. (2002) Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart 87:256–259
Pedra CAC, Pihkala J, Lee KJ, et al. (2000) Transcatheter closure of atrial septal defect using the Cardio-seal implant. Heart 84:320–326
Perry YY, Triedman JK, Gauvreau K, et al. (2000) Sudden death in patients after transcatheter device implantation for congenital heart disease. Am J Cardiol 85:992–995
Roa PS, Berger F, Rey C, et al. (2000) Results of transvenous occlusion of secundum atrial septal defects with fourth generation buttoned device: comparison with first, second and third generation devices. International Buttoned Device Trial Group. J Am Coll Cardiol 36:583–592
Rome JJ, Keane JF, Perry SB, et al. (1990) Double-umbrella closure of atrial septal defects: initial clinical applications. Circulation 82:751–758
Thanopoulos BD, Laskari CV, Tsousis GS, et al. (1998) Closure of atrial septal defects with the Amplatzer occlusion device: preliminary results. J Am Coll Cardiol 31:1110–1116
Thompson JDR, Aburawi EH, Watterson KG, et al. (2002) Surgical and transcatheter (Amplatzer) closure of atrial septal defect: a prospective comparison of results and cost. Heart 87:466–469
Vincent R, Raviele AA, Diehl HJ (2003) Single-center experience with the Helex septal occluder for closure of atrial septal defects in children. J Interv Cardiol 16:79–82
Visconti KJ, Bichell DP, Jonas RA, et al. (1999) Developmental outcome after surgical versus interventional closure of secundum atrial septal defect in children. Circulation 100(suppl):145–150
Zahn E, Latson L, Wilson N (2000) Acute and long term follow up results with the Helex septal occluder in an animal model (abstr). Paper was presented in the meeting of American College of Cardiology
Zahn EM, Wilson N, Cutright W, Latson L (2001) Development and testing of the Helex Occluder, a new expanded polytetrafluoroethylene Atrial septal defect occlusion system. Circulation 104:711–716
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Sisi, A.M., Gendi, S., Dilawar, M. et al. Helex Septal Occluder: Feasibility Study of Closure of Atrial Septal Defect. Pediatr Cardiol 29, 84–89 (2008). https://doi.org/10.1007/s00246-007-9053-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-007-9053-7